- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
Diagnostics Dispatch: Navigating the Frontiers of In-Vitro Innovations
Author: Vikas Kumar
February 28, 2024
Introduction:
In the dynamic landscape of healthcare,in-vitro diagnostics (IVD) play a pivotal role in disease detection, monitoring, and management. This rapidly evolving field encompasses a wide array of tests conducted outside the human body, offering precise and timely insights into various medical conditions. The in-vitro diagnostics market has witnessed significant growth in recent years, driven by increasing demand, technological advancements, and a rising focus on personalized medicine.
Unlock Insights: Receive a Sample Research Report on the In-Vitro Diagnostics Market: https://univdatos.com/reports/in-vitro-diagnostics-market-2?popup=report-enquiry
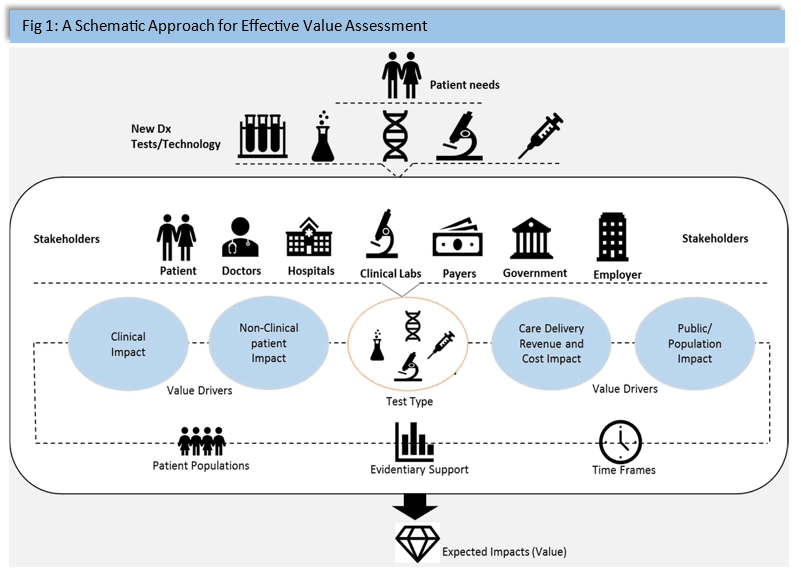
Demand:
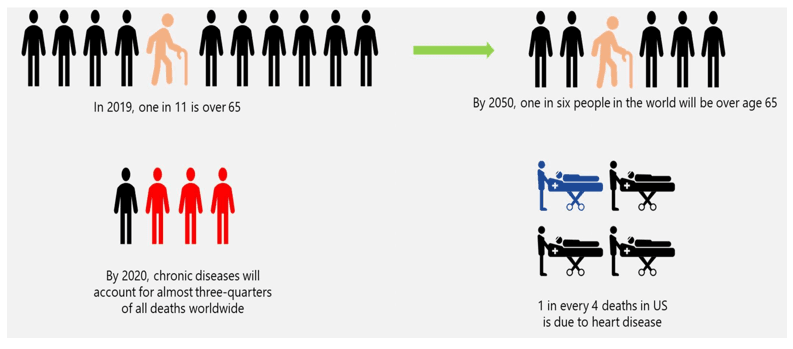
The demand for in-vitro diagnostics is propelled by several factors, including the growing prevalence of chronic diseases, an aging population, and the need for early and accurate diagnosis. Patients and healthcare professionals alike benefit from the quick and reliable results provided by IVD tests, enabling prompt decision-making regarding treatment plans. The COVID-19 pandemic has further underscored the critical role of diagnostics in public health, emphasizing the need for robust testing infrastructure and innovation.
Factors Driving the Demand of IVD:
Applications:
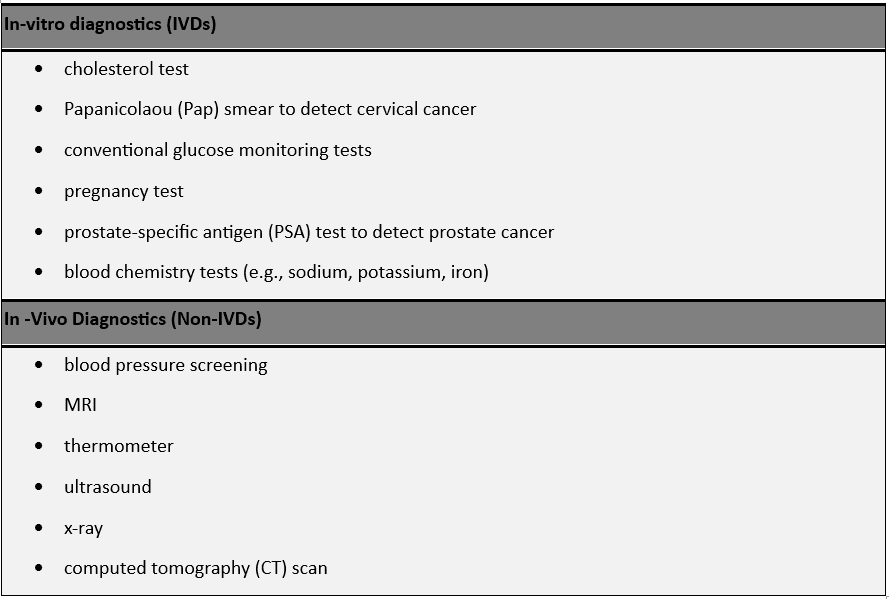
The applications of in-vitro diagnostics are diverse, covering a spectrum of medical disciplines. Clinical chemistry, immunoassays, molecular diagnostics, and hematology are among the key areas where IVD technologies are applied. From detecting infectious diseases to monitoring cardiac biomarkers, IVD has proven instrumental in tailoring treatment strategies for patients. The versatility of these diagnostic tools continues to expand, with ongoing research contributing to new applications and improved diagnostic accuracy.
Examples of Commonly Used in-vitro and in-vivo Diagnostics:-
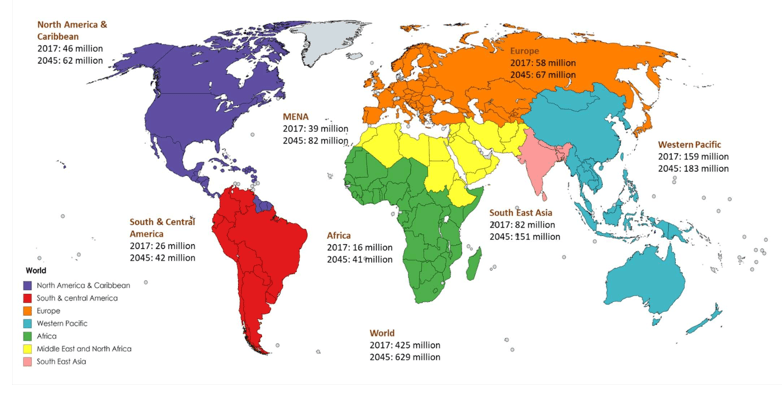
Current & Projected Number of Diabetics Globally (2017 and 2045):
Cost:
One of the challenges associated with in-vitro diagnostics is the cost of development, production, and implementation. While advancements in technology have streamlined some processes and reduced costs, certain high-tech diagnostic methods can be expensive. Striking a balance between accessibility and innovation remains a priority. Efforts are being made to enhance cost-effectiveness, ensuring that state-of-the-art diagnostics are accessible to a broader population without compromising quality.
Manufacturing:
The manufacturing landscape of in-vitro diagnostics is marked by a blend of traditional and cutting-edge techniques. Conventional methods, such as enzyme-linked immunosorbent assays (ELISA), continue to coexist with more advanced technologies likepolymerase chain reaction (PCR)and next-generation sequencing (NGS). Automation plays a crucial role in improving efficiency and reducing human error, especially in high-throughput diagnostic laboratories. Collaborations between diagnostic companies and technology providers contribute to the seamless integration of these technologies into the manufacturing process.
Explore the Comprehensive Research Overview, Including a Table of Contents, on the In-Vitro Diagnostics Market: https://univdatos.com/reports/in-vitro-diagnostics-market-2
Primary Components of IVD Processes:-
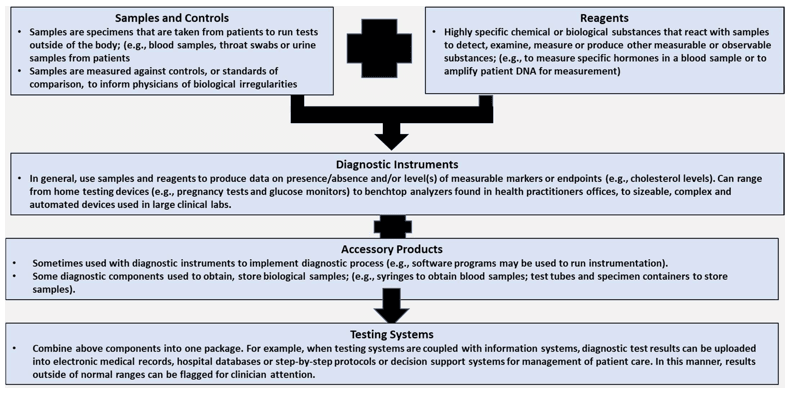
Conclusion:
In conclusion, the in-vitro diagnostics market stands at the forefront of medical innovation, driven by a growing demand for accurate and timely diagnostic solutions. The market’s trajectory is shaped by advancements in technology, expanding applications, and the ongoing pursuit of cost-effective solutions. As the healthcare landscape continues to evolve, in-vitro diagnostics will remain a cornerstone in the diagnosis and management of diseases. The commitment to accessibility, innovation, and collaboration will play a crucial role in shaping the future of this dynamic and indispensable field.
Get a Callback
