- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
ZIKA VIRUS THERAPEUTICS MARKET SEEN SOARING 4.5% GROWTH, PROJECTS UNIVDATOS
Author: Vikas Kumar
June 21, 2024
Key Highlights of the Report:
- Growing demand in the healthcare sector: The zika virus therapeutics market is experiencing significant growth due to increasing demand in the healthcare sector, driven by growing concerns over widespread mosquito-borne diseases.
- Rise in mosquito-borne disease diagnostic & treatment market: There has been a growing demand for mosquito-borne disease treatment owing to the rising prevalence of ill lifestyles.
- Rising number of Clinical Trials: Pharmaceutical giants are making progress towards developing a successful vaccine for zika virus for which clinical trials are being conducted throughout the world.
According to a new report by UnivDatos, the Zika Virus Therapeutics Market is expected to reach USD 26.4 Billion in 2030 by growing at a CAGR of 5.1%. The Zika virus is primarily transmitted through Aedes mosquito bites. It gained attention in 2015-2016 due to its link to birth defects like microcephaly in babies born to infected mothers. Research on Zika virus therapeutics is primarily focused on developing vaccines and antiviral drugs. Vaccine development aimed to create a safe and effective vaccine to prevent Zika virus infection, especially in pregnant women to protect against birth defects. Rising mosquito-borne disease cases is a major factor propelling this market. For instance, according to the Pan American Health Organization, in the Region of the Americas, in 2021, a total of 1,430,922 cases of arboviral disease were reported, of those 23,252 (1.6 %) were Zika cases. Several other factors are contributing to the growth of this market are the increased water contamination, the surge in awareness programs regarding mosquito-borne diseases and rising investments in diagnostic departments are driving the growth of the healthcare sector. Apart from these factors, rising strategic collaborations are leading the market of pharmaceutical in this forecast period.
For More Detailed Analysis in PDF Format, Visit- https://univdatos.com/reports/zika-virus-therapeutics-market?popup=report-enquiry
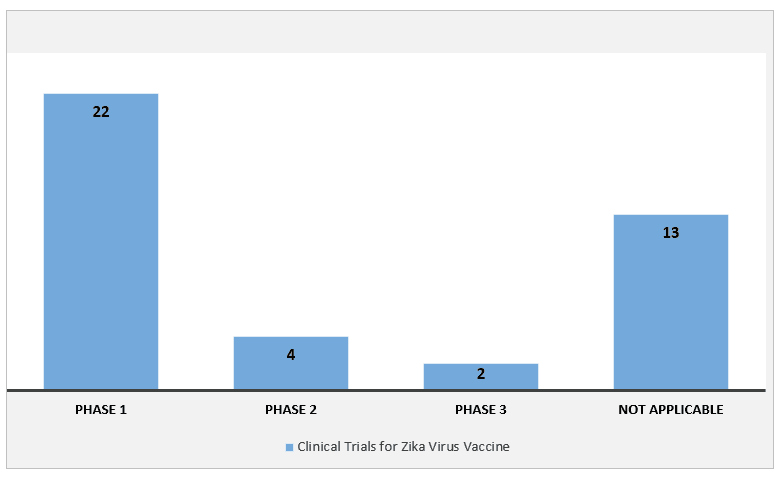
Fig: Clinical Trials for Zika Virus Vaccine, Global, 2021
The report suggests that the Rising Number of Clinical Trials is one of the major factors driving the growth of the pharmaceutical market during the forthcoming years. Multiple pharma companies are willing to spend on conducting clinical trial in hope of inventing a successful vaccine for zika virus. Money is essential for gaining prominence in the market, and the medical device industry is gaining much of it. This expenditure is driving the market for the development of new technological advancements in the field of therapeutics. In recent years, there has been an increasing number of company and government collaborations focused on zika virus therapeutics. For instance, in October 2023, the National Institute of Allergy and Infectious Diseases started a clinical trial to evaluate two zika viruses for use in controlled human infection models.It also provides hope for patients to get better care and support through zika virus therapeutics, also it can serve as a doorway to opportunities for offering better diagnostic options in the future.
Inactivated Vaccines Gaining Maximum Traction in the Market:
Inactivated vaccines, also known as killed vaccines, are made from viruses or bacteria that have been inactivated, meaning they have been killed or rendered unable to replicate. These vaccines stimulate an immune response without causing the disease itself. The process of creating inactivated vaccines involves growing large quantities of the virus or bacteria in culture and then treating them with chemicals, heat, or radiation to destroy their ability to infect cells and replicate. This ensures that the pathogen is no longer capable of causing illness, but its structure remains intact enough to trigger an immune response when introduced into the body. Because the infectious agent in these vaccines is no longer live, inactivated vaccines are considered safer for people with weakened immune systems or certain health conditions. However, they might require booster shots or adjuvants (additional substances in the vaccine) to enhance the immune response and provide long-lasting protection. Inactivated vaccines typically induce a good antibody response but may require multiple doses or booster shots to maintain immunity over time. They are an essential tool in preventing various infectious diseases and have been critical in global vaccination programs.
Conclusion
The global dual chamber pacemaker market is a rapidly growing field, with advancements in technology leading to improved outcomes for patients with the initial stage of the disease. The global zika virus therapeutics market is expected to continue to grow in the coming years, as new technologies are developed. Overall, the global zika virus therapeutics market represents a significant opportunity for pharmaceutical industries, which are making strategic alliances to design effective treatment for such deadly mosquito-borne diseases. With continued research and development, even more effective and personalized treatments will likely become available in the future, leading to improved outcomes for patients and doctors through zika virus therapeutics.
Key Offerings of the Report
Market Size, Trends, & Forecast by Revenue | 2023−2030
Market Dynamics – Leading Trends, Growth Drivers, Restraints, and Investment Opportunities
Market Segmentation – A detailed analysis by Vaccine Type, End User, and Region
Competitive Landscape – Top Key Vendors and Other Prominent Vendors
Get a Callback
