Supraventricular Tachycardia Market Seen Soaring 8.1% Growth to Reach USD 1241.61 Million by 2030, Projects Univdatos Market Insights
- Himanshu Patni
- November 17, 2023
- HEALTHCARE, NEWS
- Supraventricular Tachycardia Market, Supraventricular Tachycardia Market Share, Supraventricular Tachycardia Market Size, Supraventricular Tachycardia Market Trends
- 0 Comments
According to a new report by Univdatos Market Insights, the Supraventricular tachycardia Market is expected to reach USD 1241.61 Million in 2030 by growing at a CAGR of 9.5%. The growth or propagation of SVT often results from abnormal electrical circuits, rapid rhythms, or irregular impulses originating in different parts of the heart, creating a domino effect that can lead to sustained incidents of supraventricular tachycardia. Management and treatment strategies for SVT help to identify and interrupt these abnormal pathways to restore normal heart rhythm and prevent recurrence. Therefore, the rising cases of supraventricular tachycardia due to factors like aging populations and lifestyle-related issues are driving market growth.
Access sample report (including graphs, charts, and figures) – https://univdatos.com/get-a-free-sample-form-php/?product_id=47552
The report suggests that the Ageing Population is one of the major factors driving the supraventricular tachycardia market during the forthcoming years. The rising cases of supraventricular tachycardia due to factors like aging populations and lifestyle-related issues are driving market growth. Adding to this advances in cardiac monitoring and electrophysiology technologies improve diagnosis and treatment options. For instance, in May 2022, Bristol Myers Squibb developed mavacamten, a potential first-in-class cardiovascular medication for treatment of obstructive Hypertrophic Cardiomyopathy, a chronic heart condition with severe morbidity and patient impact, through the deal. Moreover, the growing awareness of cardiac health encourages early diagnosis and treatment of supraventricular tachycardia. Furthermore, an aging population is more prone to cardiac conditions, boosting demand for treatment options which will drive the growth of the supraventricular tachycardia market..
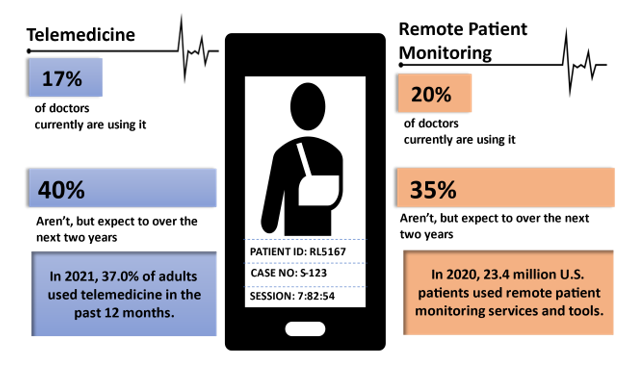
Fig1: The Rise of Telemedicine and Remote Care
Apart from this, growing technological advancements including big data analytics, internet of things, artificial intelligence, and virtual reality, amongst others in supraventricular tachycardia also positively impact the market’s growth. A wide range of investments have adopted strategic alliances in this area, thus suggesting huge potential in this area. Some of the recent strategic alliances are:
· In March 2022, Takeda Pharmaceutical Company Limited announced to acquire a portfolio of five prescription pharmaceutical products offered in China by Hasten Biopharmaceutic Co., Ltd. These five drugs include cardiovascular and metabolism drugs, which helped Hasten Biopharmaceutic Co., Ltd. to expand its portfolio.
· In September 2020, Beximco Pharmaceuticals received the approval for Flecainide Acetate, an antiarrhythmic medication, by the USFDA. The new medication was developed to treat irregular heartbeats caused by disorders such as atrial fibrillation and tachycardia.
· In June 2020, ANI Pharmaceuticals launched Mexiletine Hydrochloride Capsules USP, the company’s sixth generic product launch in 2022. These capsules are intended for treatment of potentially fatal ventricular arrhythmias.
· In May 2020, Bristol Myers Squibb and MyoKardia, Inc. signed a formal merger agreement, in which Bristol Myers Squibb paid USD13.1 billion to acquire MyoKardia.
· In May 2022, Bristol Myers Squibb, gets mavacamten, a potential first-in-class cardiovascular medication for treatment of obstructive Hypertrophic Cardiomyopathy, a chronic heart condition with severe morbidity and patient impact, through the deal.
· On January 2023, Salvat announced a study of phase 3 clinical trials for SVT-15652. In this research trial, we will conduct a multicenter, randomized, 2-arm parallel-group study on patients experiencing Otomycosis. The study will be double-blind and placebo-controlled. Our main objective is to compare the effectiveness and safety of SVT-15652 otic solution with that of a Placebo. Participants will be required to administer one vial of the assigned solution twice daily for a duration of 14 days
GOVERNMENT COMMITMENTS
On January 2023, Salvat announced a study of phase 3 clinical trials for SVT-15652. In this research trial, we will conduct a multicenter, randomized, 2-arm parallel-group study on patients experiencing Otomycosis. The study will be double-blind and placebo-controlled. Our main objective is to compare the effectiveness and safety of SVT-15652 otic solution with that of a Placebo. Participants will be required to administer one vial of the assigned solution twice daily for a duration of 14 days.
Research
On February 2023, Milestone Pharmaceuticals Inc announced a study of phase 3 clinical trials for Etripamil NS. Patients will apply the CMS themselves when they experience the onset of symptoms related to PSVT. If vagal maneuvers prove to be ineffective, patients will self-administer etripamil NS. Following an episode of PSVT where the drug is administered, patients will have the choice to return to the investigative site and opt to continue in NODE-303, utilizing etripamil NS to manage any future episodes of PSVT.
Investment
In May 2020, Bristol Myers Squibb and MyoKardia, Inc. signed a formal merger agreement, in which Bristol Myers Squibb paid USD13.1 billion to acquire MyoKardia.
Click Here To View the Report Description & TOC – https://univdatos.com/report/supraventricular-tachycardia-market/
Conclusion
The supraventricular tachycardia market is expected to experience significant growth in the coming years due to various factors such as the growth of supraventricular tachycardia management is significantly bolstered by the collaborative efforts of hospitals, clinics, ASCs, diagnostic centers, and various other healthcare stakeholders. These segments collectively ensure timely diagnosis, effective treatment, and ongoing support for individuals affected by SVT, ultimately improving patient outcomes and quality of life, which will drive the demand for the supraventricular tachycardia market over the forecast period.