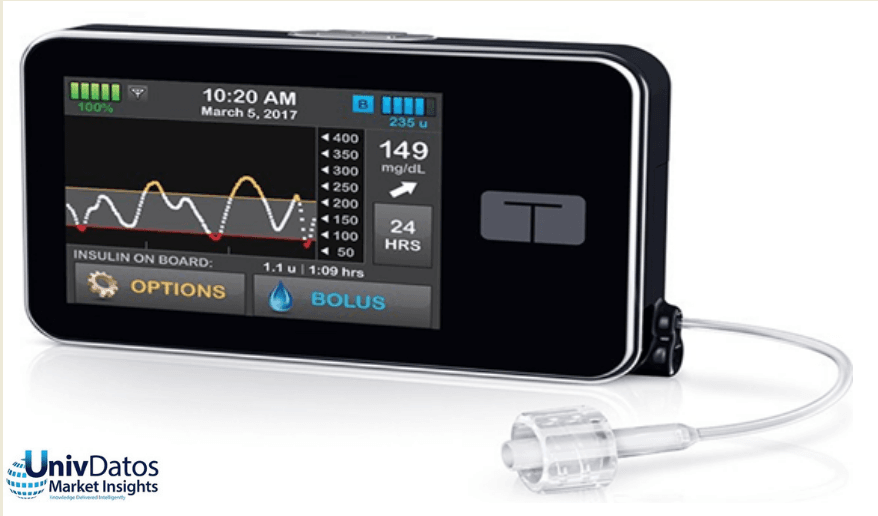
FDA 批准 Tandem 的 T:Slim X2 为首个“互操作”胰岛素泵
糖尿病是一种会损害身体处理血糖能力的疾病,是最常见且代价高昂的慢性疾病之一。对糖尿病患者的葡萄糖、血压和脂类水平进行强化治疗,可大大降低发生糖尿病相关并发症的风险。
最近在2019 年 2 月,美国食品药品监督管理局已授予 Tandem Diabetes Care Inc. 具有互操作技术的 t:Slim X2 胰岛素泵上市许可。迄今为止,胰岛素泵要么作为单一、预定义的糖尿病管理系统的一部分(III 类,最高风险设备)获得 FDA 批准,要么作为独立设备(II 类,中等风险设备)获得 FDA 批准。t:Slim X2 胰岛素泵已通过 de novo 上市前审查途径进行审查,因为它可与其他糖尿病设备组件互操作。
数字健康领域的进步使针对糖尿病护理的更多定制方法成为可能。这款 t:Slim X2 泵通过以设定的或可变的速度在皮下输送胰岛素来工作。它可以进行数字连接,并接收来自其他糖尿病管理设备的药物剂量指令。这进一步确立了 Tandem Diabetes Care 作为胰岛素泵行业主要创新者的作用。为此,它推出了首款获批为 iCGM 兼容的触摸屏泵。该泵能够通过使用个人电脑进行远程软件更新,从而使用户能够访问新功能,因为它们符合必要的监管要求。
t:slim X2 比美国所有其他耐用胰岛素泵小至少 25%,比其主要竞争对手之一提供的最新胰岛素泵外形小 38%。t:flex 提供类似时尚的泵外形,同时使用装有 480 单位胰岛素的药筒,为有更大胰岛素需求的人提供增强的灵活性。
超过三分之一的美国成年人患有糖尿病前期,并且大多数人未被诊断出来。大约有四分之一的成年人患有糖尿病,人口约为 720 万。
根据美国糖尿病协会的数据,据估计,全球有 750,000 至 100 万人使用胰岛素泵。在国内,该公司估计美国大约有 550,000 人使用胰岛素泵,其中大约 80% 患有 1 型糖尿病。这表明 t:Slim X2 泵在糖尿病市场中存在着巨大的机会。
该领域的一些主要参与者包括Roche (Accu-Chek Combo)、Insulet Corporation (Omnipod)、Medtronic (Minimed 530G System; 630G System) 和 Animas (Vibe)。
