- Inicio
- Acerca de nosotros
- Industria
- Servicios
- Leyendo
- Contáctenos
Mercado de dispositivos de valvuloplastia con balón: análisis actual y pronóstico (2023-2030)
Énfasis en el tipo (23 mm, 26 mm y 29 mm); Usuario final (Hospitales, Centros Quirúrgicos Ambulatorios y Otros); Región/País.
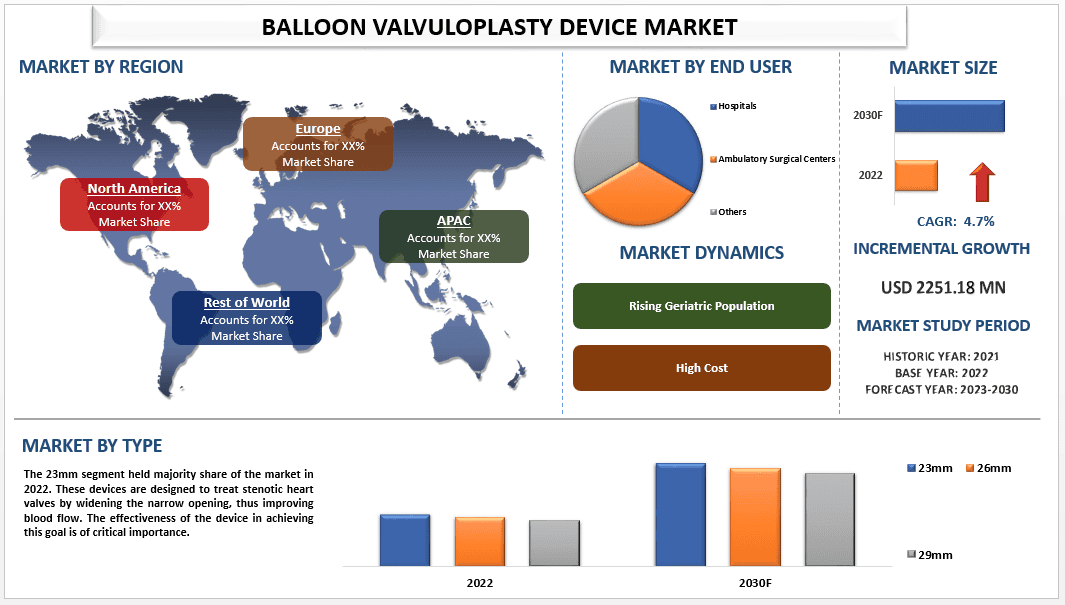
El mercado de dispositivos de valvuloplastia con balón se valoró en USD 2251,18 millones y se espera que crezca a una fuerte CAGR de alrededor del 4,7 % durante el período de pronóstico (2023-2030) debido a la creciente prevalencia de enfermedades cardiovasculares. Según los CDC, las enfermedades cardiovasculares son la principal causa de muerte en los Estados Unidos. Cada año, alrededor de 647 000 estadounidenses mueren de enfermedades cardíacas, lo que representa aproximadamente una de cada cuatro muertes. Esta estadística destaca la alarmante prevalencia de las ECV, lo que exige opciones de tratamiento eficaces como los dispositivos de valvuloplastia con balón. El NHLBI proporciona datos valiosos sobre la prevalencia de enfermedades de las válvulas cardíacas en los EE. UU. Según sus estimaciones, alrededor de 5 millones de estadounidenses padecen enfermedades de las válvulas cardíacas, incluida la estenosis valvular, lo que indica una población de pacientes importante que requiere tratamiento. Los dispositivos de valvuloplastia con balón desempeñan un papel crucial en el tratamiento de estas afecciones sin necesidad de cirugía a corazón abierto.
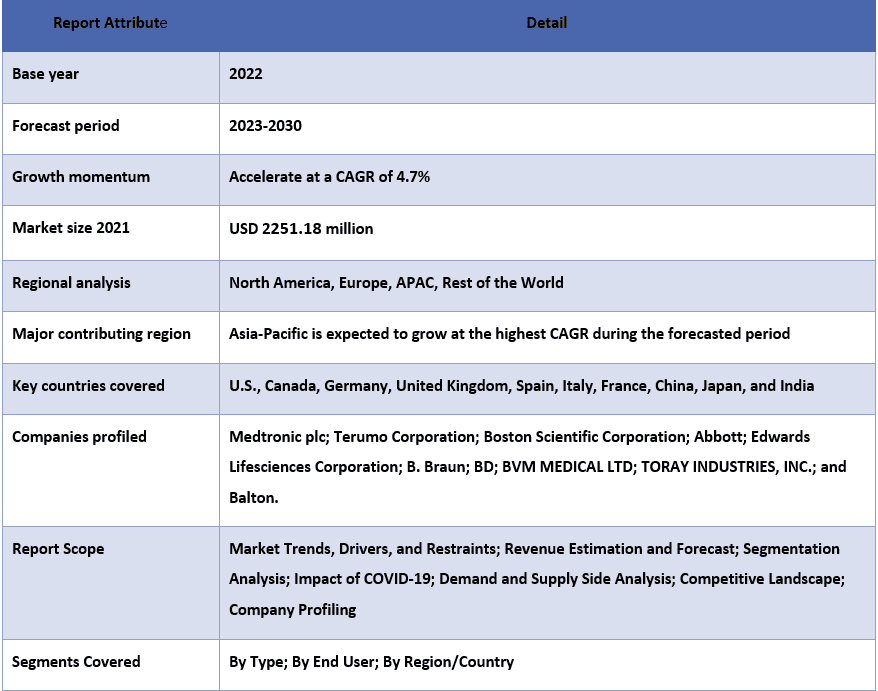
Algunos de los principales actores que operan en el mercado son Medtronic plc; Terumo Corporation; Boston Scientific Corporation; Abbott; Edwards Lifesciences Corporation; B. Braun; BD; BVM MEDICAL LTD; TORAY INDUSTRIES, INC.; y Balton. Estos actores han emprendido varias fusiones y adquisiciones junto con asociaciones para facilitar a los clientes productos/tecnologías innovadores y de alta tecnología.
Perspectivas presentadas en el informe
"Entre los tipos, se espera que la categoría de 29 mm crezca con una CAGR significativa durante el período de pronóstico"
Según el tipo, el mercado se clasifica en 23 mm, 26 mm y 29 mm. Se prevé que la categoría de 29 mm crezca con una alta CAGR durante el período de pronóstico. Los dispositivos de valvuloplastia con balón de 29 mm son dispositivos médicos que se utilizan para el tratamiento de ciertas afecciones cardíacas llamadas estenosis valvular. La estenosis valvular ocurre cuando una o más válvulas cardíacas se estrechan u obstruyen, lo que reduce el flujo sanguíneo a través del corazón. Esto puede provocar varios síntomas, como dificultad para respirar, fatiga y dolor en el pecho. El dispositivo de valvuloplastia con balón de 29 mm está diseñado específicamente para pacientes que requieren un balón de mayor diámetro para dilatar su válvula cardíaca estenótica. Es un procedimiento mínimamente invasivo que implica la inserción de un catéter con balón desinflado en la válvula afectada a través de un vaso sanguíneo, generalmente la arteria femoral en la ingle. Luego, el balón se infla para estirar y abrir la válvula estrechada, mejorando el flujo sanguíneo.
"Entre los usuarios finales, se espera que la categoría de hospitales crezca con una CAGR significativa durante el período de pronóstico"
Según el usuario final, el mercado se segmenta en hospitales, centros quirúrgicos ambulatorios y otros. Los factores impulsores para los hospitales son numerosos e incluyen la prevalencia de la enfermedad, el avance de los tratamientos y la tecnología, la calidad de la atención brindada, la disponibilidad de médicos especializados y personal de enfermería calificado, y los incentivos financieros ofrecidos por las compañías de seguros de salud. Los hospitales ofrecen tratamiento a una gran cantidad de pacientes, lo que les permite capitalizar las economías de escala y ofrecer servicios más eficientes y rentables. Los incentivos financieros de las compañías de seguros de salud también son un factor importante, ya que pueden reducir el costo del tratamiento y hacerlo más asequible para los pacientes.
Cobertura del informe del mercado de dispositivos de valvuloplastia con balón
"Se espera que APAC crezca con una alta CAGR durante el próximo período"
Se prevé que APAC crezca con una alta CAGR durante el próximo período. En primer lugar, la creciente prevalencia de enfermedades cardiovasculares, en particular las enfermedades cardíacas valvulares, está contribuyendo a la demanda de dispositivos de valvuloplastia con balón. Según la Organización Mundial de la Salud, las enfermedades cardiovasculares son la principal causa de muerte en todo el mundo, y representan casi 17,9 millones de muertes anuales. Además, la rápida aprobación del gobierno chino también está teniendo un impacto positivo en el crecimiento del mercado. Por ejemplo, en mayo de 2020, Peijia Medical recibió la aprobación de la Administración Nacional de Productos Médicos de la República Popular China para la solicitud de registro del catéter de dilatación con balón de administración Fastunnel (anteriormente denominado microcatéter de balón Neway), lo que lo convierte en el decimotercer producto neurointervencionista aprobado por la NMPA del Grupo.
Razones para comprar este informe:
- El estudio incluye análisis de dimensionamiento y pronóstico del mercado validados por expertos clave autenticados de la industria.
- El informe presenta una revisión rápida del desempeño general de la industria de un vistazo.
- El informe cubre un análisis en profundidad de los pares prominentes de la industria con un enfoque principal en las finanzas comerciales clave, las carteras de productos, las estrategias de expansión y los desarrollos recientes.
- Examen detallado de los impulsores, las restricciones, las tendencias clave y las oportunidades que prevalecen en la industria.
- El estudio cubre exhaustivamente el mercado en diferentes segmentos.
- Análisis profundo a nivel regional de la industria.
Opciones de personalización:
El mercado global de dispositivos de valvuloplastia con balón se puede personalizar aún más según el requisito o cualquier otro segmento de mercado. Además de esto, UMI entiende que puede tener sus propias necesidades comerciales, por lo tanto, no dude en conectarse con nosotros para obtener un informe que se adapte completamente a sus necesidades.
Tabla de contenido
Metodología de investigación para el análisis del mercado de dispositivos de valvuloplastia con balón (2023-2030)
El análisis del mercado histórico, la estimación del mercado actual y la previsión del mercado futuro del mercado mundial de dispositivos de valvuloplastia con balón fueron los tres pasos principales realizados para crear y analizar la adopción del dispositivo de valvuloplastia con balón en las principales regiones del mundo. Se llevó a cabo una exhaustiva investigación secundaria para recopilar las cifras del mercado histórico y estimar el tamaño actual del mercado. En segundo lugar, para validar estas conclusiones, se tuvieron en cuenta numerosos hallazgos y supuestos. Además, también se realizaron exhaustivas entrevistas primarias con expertos del sector en toda la cadena de valor del mercado mundial de dispositivos de valvuloplastia con balón. Tras la suposición y validación de las cifras del mercado a través de entrevistas primarias, empleamos un enfoque de arriba abajo/abajo arriba para pronosticar el tamaño completo del mercado. Posteriormente, se adoptaron métodos de desglose del mercado y triangulación de datos para estimar y analizar el tamaño del mercado de los segmentos y subsegmentos a los que pertenece el sector. La metodología detallada se explica a continuación:
Análisis del tamaño del mercado histórico
Paso 1: Estudio en profundidad de fuentes secundarias:
Se llevó a cabo un estudio secundario detallado para obtener el tamaño del mercado histórico del mercado de dispositivos de valvuloplastia con balón a través de fuentes internas de la empresa, como informes anuales y estados financieros, presentaciones de resultados, comunicados de prensa, etc., y fuentes externas, como revistas, noticias y artículos, publicaciones gubernamentales, publicaciones de la competencia, informes del sector, bases de datos de terceros y otras publicaciones creíbles.
Paso 2: Segmentación del mercado:
Tras obtener el tamaño del mercado histórico del mercado de dispositivos de valvuloplastia con balón, realizamos un análisis secundario detallado para recopilar información histórica del mercado y compartirla para diferentes segmentos y subsegmentos en las principales regiones. Los principales segmentos incluidos en el informe son el tipo y el usuario final. Además, se llevaron a cabo análisis a nivel de país para evaluar la adopción general de modelos de prueba en esa región.
Paso 3: Análisis de factores:
Tras adquirir el tamaño del mercado histórico de diferentes segmentos y subsegmentos, realizamos un análisis de factores detallado para estimar el tamaño actual del mercado de dispositivos de valvuloplastia con balón. Además, realizamos un análisis de factores utilizando variables dependientes e independientes, como el tipo y el usuario final del mercado de dispositivos de valvuloplastia con balón. Se llevó a cabo un análisis exhaustivo de los escenarios de oferta y demanda, teniendo en cuenta las principales asociaciones, fusiones y adquisiciones, la expansión empresarial y los lanzamientos de productos en el sector del mercado de dispositivos de valvuloplastia con balón en todo el mundo.
Estimación y previsión del tamaño actual del mercado
Tamaño actual del mercado: Basándonos en la información práctica de los 3 pasos anteriores, llegamos al tamaño actual del mercado, los principales actores del mercado mundial de dispositivos de valvuloplastia con balón y las cuotas de mercado de los segmentos. Todos los porcentajes de reparto y los desgloses del mercado necesarios se determinaron utilizando el enfoque secundario mencionado anteriormente y se verificaron a través de entrevistas primarias.
Estimación y previsión: Para la estimación y previsión del mercado, se asignaron ponderaciones a diferentes factores, incluidos los impulsores y las tendencias, las restricciones y las oportunidades disponibles para las partes interesadas. Tras analizar estos factores, se aplicaron técnicas de previsión relevantes, es decir, el enfoque de arriba abajo/abajo arriba, para llegar a la previsión del mercado para 2030 para diferentes segmentos y subsegmentos en los principales mercados a nivel mundial. La metodología de investigación adoptada para estimar el tamaño del mercado abarca:
- El tamaño del mercado del sector, en términos de ingresos (USD) y la tasa de adopción del mercado de dispositivos de valvuloplastia con balón en los principales mercados nacionales
- Todos los porcentajes de reparto, divisiones y desgloses de los segmentos y subsegmentos del mercado
- Actores clave en el mercado mundial de dispositivos de valvuloplastia con balón en términos de productos ofrecidos. Además, las estrategias de crecimiento adoptadas por estos actores para competir en el mercado de rápido crecimiento
Validación del tamaño y la cuota de mercado
Investigación primaria: Se realizaron entrevistas en profundidad con los principales líderes de opinión (KOL), incluidos los ejecutivos de alto nivel (CXO/VP, jefe de ventas, jefe de marketing, jefe de operaciones, jefe regional, jefe de país, etc.) en las principales regiones. A continuación, se resumieron las conclusiones de la investigación primaria y se realizó un análisis estadístico para demostrar la hipótesis establecida. Las aportaciones de la investigación primaria se consolidaron con las conclusiones secundarias, convirtiendo así la información en conocimientos prácticos.
División de los participantes primarios en diferentes regiones
Ingeniería de mercado
Se empleó la técnica de triangulación de datos para completar la estimación general del mercado y para llegar a cifras estadísticas precisas para cada segmento y subsegmento del mercado mundial de dispositivos de valvuloplastia con balón. Los datos se dividieron en varios segmentos y subsegmentos tras estudiar diversos parámetros y tendencias en las áreas del tipo y el usuario final en el mercado mundial de dispositivos de valvuloplastia con balón.
El principal objetivo del estudio del mercado mundial de dispositivos de valvuloplastia con balón
En el estudio se señalaron las tendencias actuales y futuras del mercado mundial de dispositivos de valvuloplastia con balón. Los inversores pueden obtener información estratégica para basar su criterio de inversión en el análisis cualitativo y cuantitativo realizado en el estudio. Las tendencias actuales y futuras del mercado determinaron el atractivo general del mercado a nivel regional, proporcionando una plataforma para que el participante industrial explote el mercado sin explotar para beneficiarse de una ventaja de ser el primero en actuar. Otros objetivos cuantitativos de los estudios incluyen:
- Analizar el tamaño actual y previsto del mercado de dispositivos de valvuloplastia con balón en términos de valor (USD). Además, analizar el tamaño actual y previsto del mercado de diferentes segmentos y subsegmentos
- Los segmentos del estudio incluyen áreas del tipo y el usuario final
- Definir y analizar el marco regulatorio para la industria de dispositivos de valvuloplastia con balón
- Analizar la cadena de valor involucrada con la presencia de varios intermediarios, junto con el análisis de los comportamientos de los clientes y la competencia de la industria
- Analizar el tamaño actual y previsto del mercado de dispositivos de valvuloplastia con balón para la región principal
- Los principales países de las regiones estudiadas en el informe incluyen Asia Pacífico, Europa, Norteamérica y el resto del mundo
- Perfiles de empresa del mercado de dispositivos de valvuloplastia con balón y las estrategias de crecimiento adoptadas por los actores del mercado para mantenerse en el mercado de rápido crecimiento
- Análisis en profundidad a nivel regional de la industria
Preguntas frecuentes Preguntas frecuentes
P1: ¿Cuál es el tamaño actual del mercado y el potencial de crecimiento del mercado global XX?
P2: ¿Cuáles son los factores impulsores del crecimiento del mercado mundial de dispositivos de valvuloplastia con balón?
P3: ¿Qué segmento tiene la mayor cuota del mercado mundial de dispositivos de valvuloplastia con balón por tipo?
P4: ¿Cuáles son las tecnologías y tendencias emergentes en el mercado mundial de dispositivos de valvuloplastia con balón?
P5: ¿Qué región dominará el mercado mundial de dispositivos de valvuloplastia con balón?
P6: ¿Quiénes son los actores clave que operan en el mercado mundial de dispositivos de valvuloplastia con balón?
Relacionados Informes
Los clientes que compraron este artículo también compraron