- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
Fabry Disease – Pipeline Analysis 2019
Global Fabry Disease – Pipeline Analysis 2019

Fabry Disease (also known as Anderson Fabry Disease) is a progressive X linked inherited genetic disorder of glycosphingolipid metabolism due to deficient or absent lysosomal α-galactosidase A activity. It is a devastating inborn error of metabolism with, particularly in the early stages, being played by cellular dysfunction and microvascular pathology being induced by lysosomal glycosphingolipid deposition. The absence or deficient activity of lysosomal exoglycohydrolase α-galactosidase A results in the progressive accumulation of globotriaosylceramide and related glycosphingolipids within lysosomes which are ubiquitous subcellular organelles.
The first clinical symptoms interfering with the child’s well-being and performance arise in childhood, typically between the ages of 3 and 10 years, and generally a few years later in girls than in boys. With age, progressive damage to vital organ systems develops in both genders leading to organ failure. End-stage renal disease and life-threatening cardiovascular or cerebrovascular complications limit life-expectancy. The clinical signs are multisystemic, heterogeneous, and progressive.
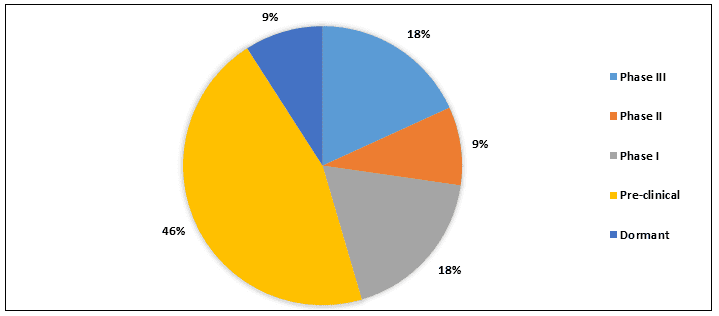
Split of Therapeutic Products
The biochemical diagnosis of Fabry Disease is established by measuring α-gal A activity in plasma or leukocytes taken from peripheral blood, cultured fibroblasts or using the samples extracted from the filter paper blood spots. The diagnosis of FD can arise from careful clinical and instrumental investigations, together with family history data and accurate interpretation of genetic and enzymatic analyses. Identification of a hemizygous GLA pathogenic variant by molecular genetic testing confirms the diagnosis in a male proband.
The therapeutic pipeline of Fabry Disease consists of approximately 9+ products in different stages of development. Currently, 2+ drugs are in Phase III development and major drugs are in pre-clinical stage.
Top Company Analysed
Some of the key players include Amicus Therapeutics; Evotec; Freeline; Greenovation Biotech; Idorsia Pharmaceuticals; Moderna; Pharming; Protalix Biotherapeutics; Resverlogix Corp; Sangamo Therapeutics and Sanofi Genzyme.
Scope of the study:
Provides an overview of therapeutic pipeline activity for Fabry Disease across the complete product development cycle including all clinical and non-clinical stages
The report comprises of detailed profiles of Fabry Disease therapeutic products with key coverage of developmental activities including licensing & collaboration deals, patents issued, designations, technologies and chemical information
Therapeutic assessment of the active pipeline products by stage, product type, molecule type, and route of administration
Detailed profiles of the dormant products have been included in the report
Reasons to buy:
The Fabry Disease pipeline presents the detailed profile of drugs. The analysis offered in the report is a combination of deep dive secondary research and input from Key Opinion Leader of the industry
The report presents a quick review of the current scenario regarding the drug development of the indication at one glance
The report covers in-depth analysis of prominent industry peers with a primary focus on company consolidation, designation, technology, agreements and patents regarding the therapy
Detailed examination on diagnosis, treatment and guidelines prevailing in the industry
Examination of industry attractiveness with the help of launch timelines
The study comprehensively covers the market across drugs in different phases of development
Extensive domain knowledge on therapy areas support the client in decision-making process regarding their therapeutic portfolio by identifying the reason behind the inactive or discontinued products
Customization Options:
The Fabry Disease pipeline analysis report can be customized to the country level or any other competitive segment. Besides this, UMI understands that you may have your own business need, hence we also provide fully customized solutions to clients.
Table of Content
Overview of pipeline development activities for Fabry Disease Pipeline Analysis of therapeutic drugs is not limited to drug description and development activities but also focuses on clinical and non-clinical results. It also includes designations, company consolidations & licensing deals, grants, technology and patent details. Therapeutic segmentation of products for Fabry Disease is done based on phase of development of the drugs. The report comprises of comparative pipeline therapeutics assessment along with detailed drug profile of pipeline products by development stage, therapy type, molecule type, and administration route across this indication. The report also consists of the launch timelines forecasted for the upcoming pipeline therapies. Additionally, Analyst Insight have also been focused upon to give a summary regarding the current market scenario. The detailed profiles of the dormant products have elucidated in the complete report with the relevant reasons for their dormancy.
Secondary Research
Detail secondary study was conducted to obtain the Fabry Disease Pipeline Analysis through company internal sources such as annual reports, performance presentations, press releases etc., and external sources including trade journals, news & articles, government publications, competitor publications, sector reports, regulatory bodies publications, safety standard organizations, third-party database and other creditable publications.
The secondary research on internal and external sources is being carried out to source qualitative and quantitative information relating to each market.
- Company websites, annual reports, quarter reports, financial reports, broker reports, investor presentations and SEC filings
- Industry trade journals and other literature
- National government documents, statistical databases and market reports
- News articles, press releases and web-casts specific to the companies operating in the market
- Several databases for patents and clinical trials
Main objective of the Fabry Disease – Pipeline Analysis 2019
The current pipeline trends of the Fabry Disease are pinpointed in the study. Investors can gain strategic insights to base their discretion for investments from the qualitative and quantitative analysis performed in the study. Current and future pipeline trends would determine the overall attractiveness of the market, providing a platform for the industrial participant to exploit the untapped market to benefit as first mover advantage. The quantitative objectives for the report include:
- An overview of therapeutic pipeline activity for Fabry Disease across the complete product development cycle including all clinical and non-clinical stages
- Analyse the detailed profiles of Fabry Disease therapeutic products with key coverage of developmental activities including licensing & collaboration deals, patents issued, designations, technologies and chemical properties information
- Therapeutic assessment of the active pipeline products by stage, product type, molecule type, and route of administration
Related Reports
Customers who bought this item also bought