- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
In-Vitro Diagnostics Market: Current Analysis and Forecast (2025-2033)
Emphasis on Product (Reagents and Instruments Services); Techniques (Immunoassay, Clinical Chemistry, Self-Monitoring of Blood Glucose (SMBG), Molecular Diagnostics, Hematology, Microbiology, Point-of-Care, and Hemostasis); Application (Infectious Disease, Diabetes, Oncology, Cardiology, Nephrology, Autoimmune Disease, and Others); End-User (Hospital In-Vitro Diagnostic, Clinics & Laboratory In-Vitro Diagnostic, Home Care In-Vitro Diagnostic, Others Healthcare Facility In-Vitro Diagnostic); Usability (Disposable IVD Devices and Reusable IVD Devices); and Region/Country
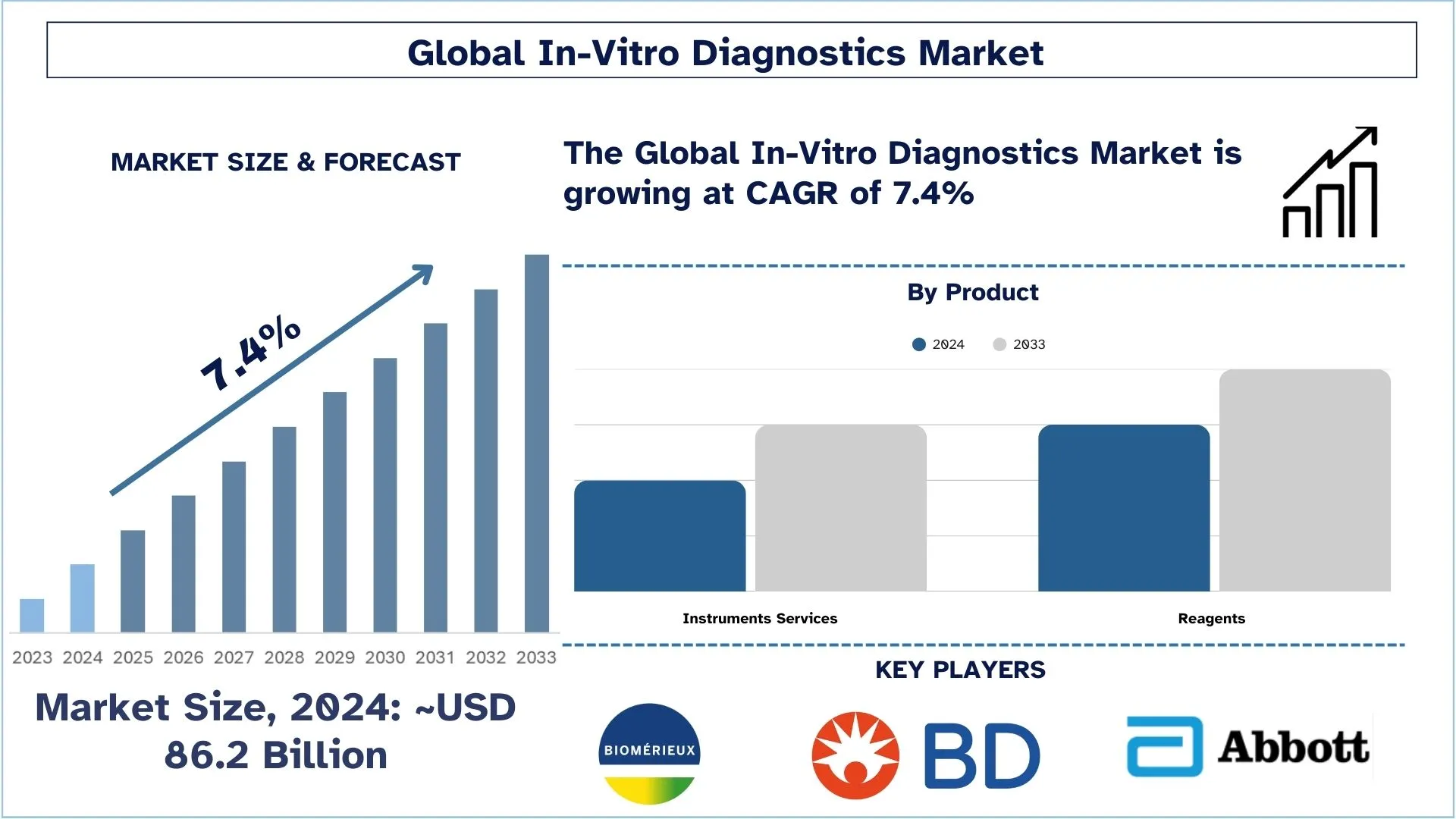
In-Vitro Diagnostics Market Size & Forecast
The In-Vitro Diagnostics Market was valued at approximately USD 86.2 billion in 2024 and is expected to grow at a substantial CAGR of around 7.4% during the forecast period (2025-2033), owing to the growing demand for decentralized and home-based diagnostic testing.
In-Vitro Diagnostics Market Analysis
In vitro diagnostics consists of medical devices and consumables that are used to perform in vitro tests on various biological samples for the detection of various medical conditions, such as diabetes and cancer. The high prevalence of chronic and infectious diseases, the rising use of point-of-care (POC) diagnostics, cutting-edge technologies in in-vitro diagnostic products, and the growing acceptance of companion diagnostics and personalized medicine are all factors contributing to the growth of the global IVD market. For instance, according to the Centers for Disease Control and Prevention (CDC), in 2021, around 18.2 million adults aged 20 and older had coronary artery disease (CAD) in the United States. Moreover, key players are rapidly launching technologically advanced products, which is also having a positive impact on market growth. For instance, in January 2021, Abbott received FDA approval for its rapid handheld Traumatic Brain Injury (TBI) blood test. The first-of-its-kind test is used for assessing mild TBIs and concussions in patients.
In-Vitro Diagnostics Market Trends
This section discusses the key market trends influencing the various segments of the In-Vitro Diagnostics market as identified by our research experts.
The rapid digitalization of diagnostics
Digital diagnostics are rapidly transforming globally because of AI and big data analytics applied with cloud connectivity in laboratory testing. The modernization of diagnostics through these developments generates faster diagnostics that produce more exact results without boundaries of location or resource constraints. The combination of AI platforms allows medical professionals to evaluate test outcomes and detect abnormalities, and forecast disease risks, so they can provide individualized healthcare at decreased healthcare costs.
In-Vitro Diagnostics Industry Segmentation
This section provides an analysis of the key trends in each segment of the global In-Vitro Diagnostics report, along with forecasts at the global, regional, and country levels for 2025-2033.
The Reagents Segment Holds the Largest Share of the Market.
Based on type, the market is segmented into reagents and instrument services. The reagents category dominates the market growth during the forecast period, owing to the extensive R&D projects being carried out by key industry participants for the development of new reagents. Companies can now concentrate on lucrative niche markets in the IVD industry thanks to the introduction of kits that enable speedier cancer detection. For instance, in February 2022, LiquidPlex Dx and FusionPlex Dx cancer diagnostic kits were launched by Invitae to enable the effective management of cancer patients with medication and prompt delivery of the necessary information.
The Immunoassay Segment is Expected to Witness a Higher CAGR than the In-Vitro Diagnostics Market.
Based on techniques, the market is categorized into immunoassay, clinical chemistry, self-monitoring of blood glucose (SMBG), molecular diagnostics, hematology, microbiology, point-of-care, and hemostasis. Among these, the immunoassay held a significant share of the market in 2021. This is mainly due to its high sensitivity analysis, high throughput, low cost, and inherent specificity in the analysis of biological samples. Also, the rising prevalence of diabetes can contribute to the growth of the market. For instance, according to the National Institutes of Health (NIH), an estimated 34.2 million people have diabetes (10.5 percent of the U.S. population).
North America has a significant share of the market in 2024.
North America is anticipated to grow at a substantial CAGR during the forecast period. The use of IVD will lead to the early diagnosis of diseases. Moreover, healthcare spending is highest in this region, driving the adoption of healthcare technology. The growing demand for in-vitro diagnostics in the region is mainly due to the increase in the aging population, escalation in the incidence and prevalence of chronic diseases, growing adoption of point-of-care diagnostics, and medical and customer needs are the major drivers for this market growth.
U.S. Dominates the North American In-Vitro Diagnostics Market
The U.S. IVD market demonstrates the largest and most advanced configuration among global diagnostic markets due to excellent healthcare capabilities coupled with powerful diagnostic corporations and intensive needs for early disease monitoring and individualized medical approaches. The IVD market continues developing because of molecular diagnostic trends coupled with rising chronic disease rates and ongoing funding of in-vitro diagnostics used at points of care and in home settings. Market expansion and innovation receive further support because of regulatory approval from the FDA alongside growing implementation of diagnostic systems which integrate AI and data analytics technologies.
In-Vitro Diagnostics Industry Competitive Landscape
The In-Vitro Diagnostics market is competitive, with several global and international players. The key players are adopting different growth strategies to enhance their market presence, such as partnerships, agreements, collaborations, new product launches, geographical expansions, and mergers and acquisitions.
Top In-Vitro Diagnostics Companies
Some of the major players operating in the market are Abbott, BD; BIOMÉRIEUX; Bio-Rad Laboratories, Inc.; Danaher; Johnson & Johnson Private Limited; F. Hoffmann-La Roche Ltd; Siemens Healthcare GmbH; Sysmex Corporation; Thermo Fisher Scientific
Recent Developments in the In-Vitro Diagnostics Market
February 2024 – Thermo Fisher Scientific Inc. launched a new ion chromatography instrument to improve the reliability, efficiency, and functional adaptability of labs.
December 2023 – Thermo Fisher Scientific Inc. signed a distribution agreement with AESKU GROUP GmbH, a provider of innovative diagnostic solutions, to market IFA testing kits and instruments in the U.S.
December 2023 – Sysmex Corporation received insurance coverage for its immunoassay reagent HISCL M2BPGi-Qt Assay Kit to cater to the growing cases of chronic hepatitis.
In-Vitro Diagnostics Market Report Coverage
Report Attribute | Details |
Base year | 2024 |
Forecast period | 2025-2033 |
Growth momentum | Accelerate at a CAGR of 7.4% |
Market size 2024 | USD 86.2 Billion |
Regional analysis | APAC, Europe, Asia-Pacific, Rest of the World |
Major contributing region | North America is expected to grow at the highest CAGR during the forecasted period. |
Key countries covered | U.S., Canada, Germany, France, UK, Spain, Italy, China, Japan, and India |
Abbott; BD; BIOMÉRIEUX; Bio-Rad Laboratories, Inc.; Danaher; Johnson & Johnson Private Limited; F. Hoffmann-La Roche Ltd; Siemens Healthcare GmbH; Sysmex Corporation; Thermo Fisher Scientific | |
Report Scope | Market Trends, Drivers, and Restraints; Revenue Estimation and Forecast; Segmentation Analysis; Demand and Supply Side Analysis; Competitive Landscape; Company Profiling |
Segments Covered | By Product, By Technique, By Application, By End-User, By Region/Country |
Reasons to Buy the In-Vitro Diagnostics Market Report:
The study includes market sizing and forecasting analysis validated by authenticated key industry experts.
The report presents a quick review of overall industry performance at a glance.
The report covers an in-depth analysis of prominent industry peers with a primary focus on key business financials, product portfolios, expansion strategies, and recent developments.
Detailed examination of drivers, restraints, key trends, and opportunities prevailing in the industry.
The study comprehensively covers the market across different segments.
Deep dive regional-level analysis of the industry.
Customization Options:
The global In-Vitro Diagnostics market can be customized further as per the requirements or any other market segment. Besides this, UnivDatos understands that you may have your own business needs; hence, feel free to contact us to get a report that completely suits your requirements.
Table of Content
Research Methodology for In-Vitro Diagnostics Market Analysis (2023-2033)
We analyzed the historical market, estimated the current market, and forecasted the future market of the global In-Vitro Diagnostics market to assess its application in major regions worldwide. We conducted exhaustive secondary research to gather historical market data and estimate the current market size. To validate these insights, we carefully reviewed numerous findings and assumptions. Additionally, we conducted in-depth primary interviews with industry experts across the In-Vitro Diagnostics value chain. After validating market figures through these interviews, we used top-down and bottom-up approaches to forecast the overall market size. We then employed market breakdown and data triangulation methods to estimate and analyze the market size of industry segments and sub-segments.
Market Engineering
We employed data triangulation techniques to finalize the overall market estimation and derive precise statistical numbers for each segment and sub-segment of the global In-Vitro Diagnostics market. We split the data into several segments and sub-segments by analyzing various parameters and trends, including product, technique, application, end-user, and regions within the global In-Vitro Diagnostics market.
The main objective of the Global In-Vitro Diagnostics Market Study
The study identifies current and future trends in the global In-Vitro Diagnostics market, providing strategic insights for investors. It highlights regional market attractiveness, enabling industry participants to tap into untapped markets and gain a first-mover advantage. Other quantitative goals of the studies include:
- Market Size Analysis: Assess the current and forecast market size of the global In-Vitro Diagnostics market and its segments in terms of value (USD).
- IN-VITRO DIAGNOSTICS Market Segmentation: The study segments the market by product, technique, application, end-user, and region.
- Regulatory Framework & Value Chain Analysis: Examine the regulatory framework, value chain, customer behavior, and competitive landscape of the In-Vitro Diagnostics industry.
- Regional Analysis: Conduct a detailed regional analysis for key areas such as Asia Pacific, Europe, North America, and the Rest of the World.
- Company Profiles & Growth Strategies: Company profiles of the In-Vitro Diagnostics market and the growth strategies adopted by the market leaders to sustain the fast-growing market.
Frequently Asked Questions FAQs
Q1: What is the In-Vitro Diagnostics market's current size and growth potential?
As of 2024, the global In-Vitro Diagnostics market is valued at approximately USD 86.2 billion and is projected to grow at a CAGR of 7.4% through 2033.
Q2: What are the driving factors for the growth of the In-Vitro Diagnostics market?
Rising prevalence of infectious and chronic diseases worldwide.
Q3: Which market has the largest share of the In-Vitro Diagnostics market by Product?
The reagents segment dominates the global in vitro diagnostics market by product segment.
Q4: What are the major trends in the In-Vitro Diagnostics market?
Growing demand for decentralized and home-based diagnostic testing.
Q5: Which region will dominate the In-Vitro Diagnostics market?
The North America region currently dominates the global In-Vitro Diagnostics market.
Q6: What are the biggest challenges in the In-Vitro Diagnostics market?
Regulatory complexities and data privacy concerns with digital diagnostic tools.
Q7: Who are the Top players in the global In-Vitro Diagnostics market?
The leading companies driving innovation in In-Vitro Diagnostics include:
• Abbott
• BD
• BIOMÉRIEUX
• Bio-Rad Laboratories, Inc.
• Danaher
• Johnson & Johnson Private Limited
• F. Hoffmann-La Roche Ltd
• Siemens Healthcare GmbH
• Sysmex Corporation
• Thermo Fisher Scientific
Q8: What are the key growth opportunities for businesses in the global IVD market?
Businesses can tap into major growth opportunities by expanding into emerging markets, investing in molecular and point-of-care diagnostics, and developing AI-powered diagnostic platforms. Collaborations with healthcare providers and digital health startups can also unlock new revenue streams, especially as demand rises for faster, more accurate, and patient-friendly diagnostic solutions.
Q9: How is regulatory innovation impacting the development of new IVD technologies?
Regulatory agencies like the FDA and EMA are adopting more flexible and expedited approval pathways, especially for high-need areas such as infectious disease testing and personalized medicine. This is encouraging faster commercialization of novel diagnostics while ensuring safety and efficacy, creating an environment ripe for innovation and strategic investment.
Related Reports
Customers who bought this item also bought










