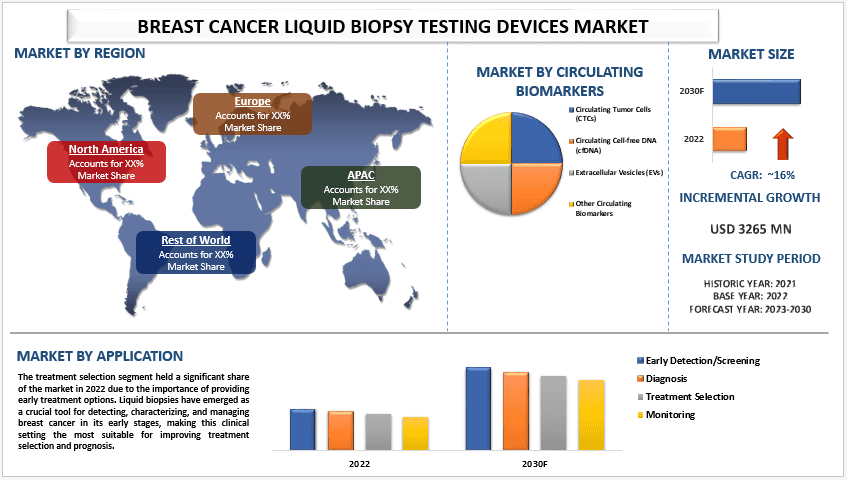
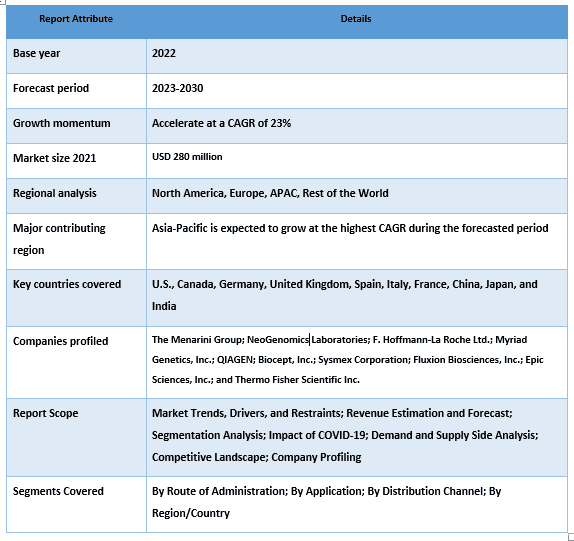
乳腺癌液体活检检测设备市场价值 32.65 亿美元,预计在预测期内(2023-2030 年)将以约 16% 的强劲复合年增长率增长。 液体活检是乳腺癌诊断和治疗的宝贵工具。液体活检的突变分析可以帮助根据患者肿瘤中存在的特定突变,确定适合患者的靶向治疗。例如,对于转移性乳腺癌患者,检测 PIK3CA 突变(约占所有 HR 阳性肿瘤的 40%),可以帮助确定最有效的治疗方案。此外,通过液体活检中循环肿瘤 DNA 检测微小残留病 (MRD) 在肿瘤学中具有广阔且变革性的应用前景。此外,监管批准的增加预计将对市场产生积极影响。例如,2019 年 6 月,美国食品和药物管理局 (FDA) 批准了 QIAGEN 的 therascreen PIK3CA RGQ PCR 试剂盒。该批准授予该设备用作伴随诊断测定,以检测乳腺癌组织和液体活检中的 PIK3CA 突变。
在The Menarini Group; NeoGenomics Laboratories; F. Hoffmann-La Roche Ltd.; Myriad Genetics, Inc.; QIAGEN; Biocept, Inc.; Sysmex Corporation; Fluxion Biosciences, Inc.; Epic Sciences, Inc.; 和 Thermo Fisher Scientific Inc.等公司中,一些主要参与者正在运营。这些参与者已经进行了多次并购以及合作,以便为客户提供高科技和创新产品/技术。
报告中提出的见解
“在循环生物标志物中,循环肿瘤细胞细分市场预计在预测期内将以高复合年增长率增长”
根据循环生物标志物,市场分为循环肿瘤细胞 (CTCs)、循环游离 DNA (cfDNA)、细胞外囊泡 (EVS) 和其他循环生物标志物。预计循环肿瘤细胞 (CTCs) 在预测期内将以显着的复合年增长率增长。随着 CTC 监测的进展,它们的水平可以提供对治疗效果的洞察。最近的研究探索了 CTC 作为早期癌症检测生物标志物的潜力,较高的 CTC 计数表明发展为癌症的风险较高。早期乳腺癌中 CTC 的检测一直是正在进行的研究的重点,FDA 批准的 CellSearch 系统被用于从外周血样本中分离 CTC。近年来,在提高 CTC 分离技术的效率和准确性方面取得了重大进展,为未来更准确地检测和监测癌症带来了希望。
“在应用中,治疗选择类别在 2022 年占据了重要的市场份额”
按应用,市场分为早期检测/筛查、诊断、治疗选择和监测。由于提供早期治疗选择的重要性,治疗选择细分市场在 2022 年占据了重要的市场份额。液体活检已成为检测、表征和管理早期乳腺癌的关键工具,使这种临床环境最适合改善治疗选择和预后。例如,2020 年 8 月,FoundationOne Liquid CDx 获得了 FDA 批准,其基于 ctDNA 的 CGP 测定旨在与其他诊断测试结合使用,以帮助医生确定哪些患者将受益于接受靶向药物。
“亚太地区预计在预测期内将以高复合年增长率增长”
由于医疗改革、医疗基础设施的改善、人口增长以及更多公司进入市场等多种因素,预计亚太地区在预测期内将经历显着增长。根据全球癌症统计 2020,亚太地区人口众多且癌症患病率高,据估计 2020 年亚洲发生了 58.3% 的癌症死亡,这使得癌症筛查成为该地区日益重要的优先事项。因此,近年来,癌症筛查测试的使用有所增加,各国政府正在实施免费乳腺癌筛查等举措,公司也在合作以增加这些测试的分配和供应。例如,2021 年,中国初创公司 AnchorDx 与中国一家顶级医院合作开发了一种基于 cfDNA 的液体活检测试,用于早期检测乳腺癌。
乳腺癌液体活检检测设备市场报告覆盖范围
购买本报告的理由:
- 该研究包括市场规模和预测分析,并经过认证的关键行业专家的验证。
- 该报告以一目了然的方式快速回顾了整体行业绩效。
- 该报告涵盖了对主要行业同行的深入分析,主要侧重于关键业务财务、产品组合、扩张战略和最新发展。
- 详细审查行业中普遍存在的驱动因素、限制因素、关键趋势和机遇。
- 该研究全面涵盖了跨不同细分市场的市场。
- 对行业进行深入的区域层面分析。
定制选项:
全球乳腺癌液体活检检测设备市场可以根据需求或任何其他细分市场进一步定制。除此之外,UMI 了解到您可能有自己的业务需求,因此请随时与我们联系以获取完全适合您需求的报告。
目录
乳腺癌液体活检检测设备市场分析(2023-2030)的研究方法
分析历史市场、评估当前市场以及预测全球乳腺癌液体活检检测设备市场的未来市场,是创建和分析全球主要地区乳腺癌液体活检检测设备采用情况所采取的三个主要步骤。进行了详尽的二级研究,以收集历史市场数据并评估当前市场规模。其次,为了验证这些见解,考虑了大量的发现和假设。此外,还与全球乳腺癌液体活检检测设备市场价值链中的行业专家进行了详尽的初步访谈。在通过初步访谈假设和验证市场数据后,我们采用了自上而下/自下而上的方法来预测完整的市场规模。此后,采用市场细分和数据三角测量方法来评估和分析行业相关细分市场和子细分市场的市场规模。详细方法如下所述:
历史市场规模分析
第一步:深入研究二级来源:
进行了详细的二级研究,以通过公司内部来源(例如年度报告和财务报表、业绩演示文稿、新闻稿等)以及外部来源(包括期刊、新闻和文章、政府出版物、竞争对手出版物、行业报告、第三方数据库和其他可靠出版物)来获取乳腺癌液体活检检测设备市场的历史市场规模。
第二步:市场细分:
在获得乳腺癌液体活检检测设备市场的历史市场规模后,我们进行了详细的二级分析,以收集主要地区不同细分市场和子细分市场的历史市场见解和份额。报告中包含的主要细分市场为循环生物标志物和应用。此外,还进行了国家/地区层面的分析,以评估该地区检测模型的总体采用情况。
第三步:因素分析:
在获得不同细分市场和子细分市场的历史市场规模后,我们进行了详细的因素分析,以评估乳腺癌液体活检检测设备市场的当前市场规模。此外,我们使用循环生物标志物和乳腺癌液体活检检测设备市场的应用等因变量和自变量进行了因素分析。考虑到全球乳腺癌液体活检检测设备市场领域的顶级合作伙伴关系、并购、业务扩张和产品发布,对需求和供应侧情景进行了全面分析。
当前市场规模评估和预测
当前市场规模评估:根据上述 3 个步骤的可行见解,我们得出了全球乳腺癌液体活检检测设备市场的当前市场规模、主要参与者以及细分市场的市场份额。所有必需的百分比份额拆分和市场细分均使用上述二级方法确定,并通过初步访谈进行了验证。
评估与预测:对于市场评估和预测,对不同因素(包括驱动因素和趋势、限制因素以及利益相关者的可用机会)分配了权重。在分析这些因素后,应用相关的预测技术,即自上而下/自下而上的方法,以得出全球主要市场不同细分市场和子细分市场的 2030 年市场预测。用于评估市场规模的研究方法包括:
- 该行业的市场规模,以收入(美元)和乳腺癌液体活检检测设备市场在国内主要市场的采用率衡量
- 市场细分和子细分的全部百分比份额、拆分和细分
- 全球乳腺癌液体活检检测设备市场中主要参与者提供的产品。此外,这些参与者为在快速增长的市场中竞争而采取的增长战略
市场规模和份额验证
一级研究:与主要意见领袖 (KOL)(包括主要地区的顶级高管(CXO/VP、销售主管、营销主管、运营主管、区域主管、国家/地区主管等))进行了深入访谈。然后总结一级研究结果,并进行统计分析以证明既定假设。一级研究的输入与二级研究结果合并,从而将信息转化为可行的见解。
不同地区一级参与者的细分
市场工程
采用数据三角测量技术来完成整体市场评估,并得出全球乳腺癌液体活检检测设备市场每个细分市场和子细分市场的精确统计数据。在研究了全球乳腺癌液体活检检测设备市场中循环生物标志物和应用领域的各种参数和趋势后,将数据分为几个细分市场和子细分市场。
全球乳腺癌液体活检检测设备市场研究的主要目标
该研究指出了全球乳腺癌液体活检检测设备市场的当前和未来市场趋势。投资者可以获得战略见解,从而根据研究中执行的定性和定量分析来确定其投资的依据。当前和未来的市场趋势决定了区域层面的市场整体吸引力,为行业参与者提供了一个利用未开发市场以从先发优势中获益的平台。研究的其他定量目标包括:
- 分析乳腺癌液体活检检测设备市场当前和预测的市场规模,以价值(美元)衡量。此外,分析不同细分市场和子细分市场的当前和预测的市场规模
- 研究中的细分市场包括循环生物标志物和应用领域
- 定义和分析乳腺癌液体活检检测设备行业的监管框架
- 分析涉及各种中介机构的价值链,同时分析行业的客户和竞争对手行为
- 分析主要区域乳腺癌液体活检检测设备市场当前和预测的市场规模
- 报告中研究的区域的主要国家包括亚太地区、欧洲、北美和世界其他地区
- 乳腺癌液体活检检测设备市场的公司概况以及市场参与者为在快速增长的市场中维持发展而采取的增长战略
- 深入分析行业的区域层面
相关 报告
购买此商品的客户也购买了