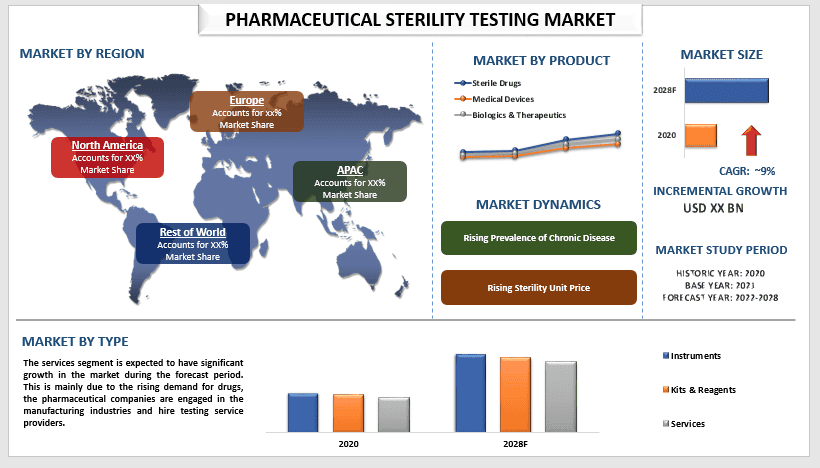
전 세계 제약 무균성 테스트 시장은 예측 기간 동안 약 9%의 상당한 성장률을 보일 것으로 예상됩니다. 살균은 가장 저항력이 강한 박테리아와 포자를 포함한 모든 미생물의 통계적으로 완전한 파괴로 정의될 수 있습니다. 인구 증가와 질병 유병률 증가로 인해 효과적인 의약품에 대한 수요가 지속적으로 증가하는 것은 제약 무균성 테스트 성장의 중요한 요인입니다. 예를 들어 2019년에는 미국인 3,730만 명, 즉 인구의 11.3%가 당뇨병을 앓고 있었습니다. 또한 미생물 통제 및 우수 제조 공정에 대한 엄격한 정부 규제도 제약 무균성 테스트 산업의 시장 성장에 기여합니다.
Pacific Biolabs, STRERIS Corporation, Boston Analytical, Gibraltar Laboratories, Sartorius AG, SolviasAG, SGS AG, Toxikon inc., Merck KGaA, Charles River Laboratories International Inc. 등이 시장의 주요 업체입니다. 하이테크 및 혁신적인 제품/기술로 고객을 지원하기 위해 이러한 업체들은 여러 M&A와 파트너십을 체결했습니다.
보고서에 제시된 인사이트
"샘플 중 멸균 약물 범주가 예측 기간 동안 더 높은 CAGR을 기록할 것입니다."
샘플을 기준으로 시장은 멸균 약물, 의료 기기, 생물학적 제제 및 치료제로 분류되었습니다. 멸균 약물 범주는 예측 기간 동안 더 높은 CAGR을 기록할 것으로 예상됩니다. 이는 주로 이러한 약물이 내부 신체 기관과 직접 접촉하고 오염된 약물의 여파가 심각할 수 있기 때문입니다. 또한 체내 흡수율이 높은 주사제에 대한 수요가 증가하는 것도 멸균 약물의 부문별 성장에 기여합니다.
"유형 중 서비스가 2020년에 시장에서 상당한 점유율을 차지할 것입니다."
유형을 기준으로 시장은 기기, 키트 및 시약, 서비스로 분류되었습니다. 그중 서비스 부문이 시장에서 상당한 성장을 이룰 것으로 예상됩니다. 기업들은 또한 무균성 테스트 시장에서 새로운 서비스를 제공하여 시장의 부문별 성장을 촉진하고 있습니다. 예를 들어 2019년 10월 Alcami Corporation은 최첨단 미생물학 테스트 기술을 활용하여 고객에게 훨씬 더 빠른 테스트 결과를 제공하는 신속한 무균성 제품인 서비스를 출시했습니다.
"북미가 시장에서 상당한 점유율을 차지할 것입니다."
제약 무균성 테스트 산업의 시장 채택에 대한 더 나은 이해를 위해 시장은 북미 (미국, 캐나다 및 북미 나머지 지역), 유럽 (독일, 프랑스, 이탈리아, 스페인, 영국 및 유럽 나머지 지역), 아시아 태평양 (중국, 일본, 인도, 호주 및 APAC 나머지 지역) 및 세계 나머지 지역과 같은 국가에서 전 세계적으로 존재 여부에 따라 분석됩니다. 2020년에 북미는 전 세계 제약 무균성 테스트 시장에서 상당한 점유율을 차지했습니다. 이는 주로 우수 제조 공정에 대한 엄격한 지침과 함께 의료 및 제약 부문에 대한 높은 투자에 기인합니다. 예를 들어 정부의 과학 동원 계획에서 코로나바이러스 연구 및 의료 대응책에 대한 1억 9,200만 달러
이 보고서를 구매해야 하는 이유:
- 이 연구에는 인증된 주요 산업 전문가가 검증한 시장 규모 측정 및 예측 분석이 포함되어 있습니다.
- 이 보고서는 전체 산업 성과에 대한 간략한 검토를 한눈에 제공합니다.
- 이 보고서는 주요 비즈니스 재무, 제품 포트폴리오, 확장 전략 및 최근 개발에 중점을 둔 주요 산업 동료에 대한 심층 분석을 다룹니다.
- 산업 전반에 걸쳐 존재하는 동인, 제약, 주요 추세 및 기회에 대한 자세한 조사.
- 이 연구는 다양한 부문에 걸쳐 시장을 포괄적으로 다룹니다.
- 산업에 대한 심층적인 지역 수준 분석.
맞춤화 옵션:
전 세계 제약 무균성 테스트 시장은 요구 사항 또는 기타 시장 부문에 따라 추가로 맞춤화할 수 있습니다. 이 외에도 UMI는 귀하가 귀하의 비즈니스 요구 사항을 가지고 있을 수 있음을 이해하므로 귀하의 요구 사항에 완벽하게 맞는 보고서를 얻으려면 언제든지 저희에게 연락하십시오.
목차
제약 무균 시험 시장 분석 (2022-2028)을 위한 연구 방법론
글로벌 제약 무균 시험 시장의 과거 시장 분석, 현재 시장 추정, 미래 시장 예측은 전 세계 주요 지역에서 제약 무균 시험 채택을 생성하고 분석하기 위해 수행된 세 가지 주요 단계였습니다. 과거 시장 수치를 수집하고 현재 시장 규모를 추정하기 위해 광범위한 2차 조사가 수행되었습니다. 둘째, 이러한 통찰력을 검증하기 위해 수많은 결과 및 가정이 고려되었습니다. 또한, 글로벌 제약 무균 시험 시장의 가치 사슬 전반에 걸쳐 업계 전문가와 함께 광범위한 1차 인터뷰도 수행되었습니다. 1차 인터뷰를 통해 시장 수치를 가정하고 검증한 후, 전체 시장 규모를 예측하기 위해 하향식/상향식 접근 방식을 사용했습니다. 그 후, 시장 세분화 및 데이터 삼각 측량 방법을 채택하여 산업 관련 세그먼트 및 하위 세그먼트의 시장 규모를 추정하고 분석했습니다. 자세한 방법론은 아래에 설명되어 있습니다.
과거 시장 규모 분석
1단계: 2차 자료 심층 연구:
연례 보고서 및 재무 제표, 실적 발표, 보도 자료 등과 같은 회사 내부 자료와 저널, 뉴스 및 기사, 정부 간행물, 경쟁사 간행물, 부문 보고서, 제3자 데이터베이스 및 기타 신뢰할 수 있는 간행물을 포함한 외부 자료를 통해 제약 무균 시험 시장의 과거 시장 규모를 확보하기 위해 자세한 2차 연구가 수행되었습니다.
2단계: 시장 세분화:
제약 무균 시험 시장의 과거 시장 규모를 확보한 후, 주요 지역에 대한 다양한 세그먼트 및 하위 세그먼트에 대한 과거 시장 통찰력과 점유율을 수집하기 위해 자세한 2차 분석을 수행했습니다. 주요 세그먼트는 세그먼트 및 유형으로 보고서에 포함되어 있습니다. 또한, 해당 지역에서 시험 모델의 전반적인 채택을 평가하기 위해 국가 수준 분석이 수행되었습니다.
3단계: 요인 분석:
다양한 세그먼트 및 하위 세그먼트의 과거 시장 규모를 확보한 후, 제약 무균 시험 시장의 현재 시장 규모를 추정하기 위해 자세한 요인 분석을 수행했습니다. 또한, 제약 무균 시험의 샘플 및 유형과 같은 종속 변수 및 독립 변수를 사용하여 요인 분석을 수행했습니다. 전 세계 제약 무균 시험 시장 부문에서 최고의 파트너십, 합병 및 인수, 사업 확장, 제품 출시를 고려하여 수요 및 공급 측면 시나리오에 대한 철저한 분석이 수행되었습니다.
현재 시장 규모 추정 및 예측
현재 시장 규모 측정: 위의 3단계에서 얻은 실행 가능한 통찰력을 바탕으로 현재 시장 규모, 글로벌 제약 무균 시험 시장의 주요 업체, 세그먼트의 시장 점유율에 도달했습니다. 필요한 모든 백분율 점유율 분할 및 시장 세분화는 위에서 언급한 2차 접근 방식을 사용하여 결정되었으며 1차 인터뷰를 통해 검증되었습니다.
추정 및 예측: 시장 추정 및 예측을 위해 동인 및 추세, 제약, 이해 관계자가 사용할 수 있는 기회를 포함한 다양한 요인에 가중치가 부여되었습니다. 이러한 요인을 분석한 후, 관련 예측 기법, 즉 하향식/상향식 접근 방식이 적용되어 전 세계 주요 시장에서 다양한 세그먼트 및 하위 세그먼트에 대한 2028년 시장 예측에 도달했습니다. 시장 규모를 추정하기 위해 채택된 연구 방법론은 다음과 같습니다.
- 매출 (USD) 측면에서 산업의 시장 규모와 국내 주요 시장에서 제약 무균 시험 시장의 채택률
- 시장 세그먼트 및 하위 세그먼트의 모든 백분율 점유율, 분할 및 세분화
- 제공되는 제품 측면에서 글로벌 제약 무균 시험 시장의 주요 업체. 또한, 빠르게 성장하는 시장에서 경쟁하기 위해 이러한 업체가 채택한 성장 전략
시장 규모 및 점유율 검증
1차 연구: 주요 지역에서 최고 경영진 (CXO/VP, 영업 책임자, 마케팅 책임자, 운영 책임자, 지역 책임자, 국가 책임자 등)을 포함한 핵심 오피니언 리더 (KOL)와 심층 인터뷰를 실시했습니다. 그런 다음 1차 연구 결과를 요약하고, 제시된 가설을 증명하기 위해 통계 분석을 수행했습니다. 1차 연구의 입력은 2차 결과와 통합되어 정보를 실행 가능한 통찰력으로 전환했습니다.
다양한 지역에서 1차 참가자 분할
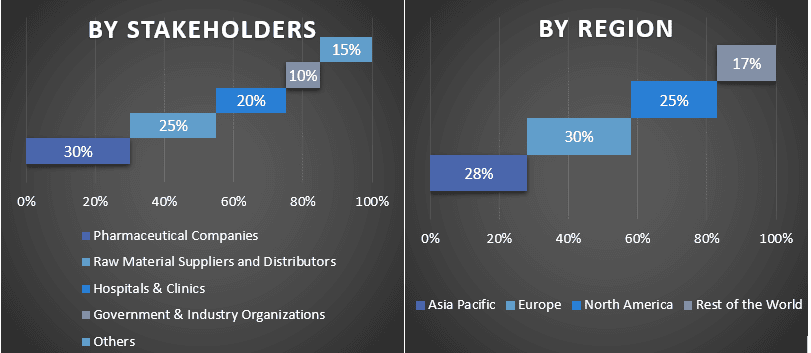
시장 엔지니어링
데이터 삼각 측량 기법을 사용하여 전체 시장 추정을 완료하고 글로벌 제약 무균 시험 시장의 각 세그먼트 및 하위 세그먼트에 대한 정확한 통계 수치에 도달했습니다. 글로벌 제약 무균 시험 시장에서 샘플 및 유형 분야의 다양한 매개 변수 및 추세를 연구한 후 데이터를 여러 세그먼트 및 하위 세그먼트로 분할했습니다.
글로벌 제약 무균 시험 시장 조사의 주요 목표
글로벌 제약 무균 시험 시장의 현재 및 미래 시장 동향이 연구에서 정확히 지적되었습니다. 투자자는 연구에서 수행된 질적 및 양적 분석을 기반으로 투자에 대한 재량을 가질 수 있는 전략적 통찰력을 얻을 수 있습니다. 현재 및 미래 시장 동향은 지역 수준에서 시장의 전반적인 매력을 결정하여 산업 참가자가 미개발 시장을 활용하여 선점자 이점을 얻을 수 있는 플랫폼을 제공했습니다. 연구의 다른 정량적 목표는 다음과 같습니다.
- 가치 (USD) 측면에서 제약 무균 시험 시장의 현재 및 예측 시장 규모를 분석합니다. 또한, 다양한 세그먼트 및 하위 세그먼트의 현재 및 예측 시장 규모를 분석합니다.
- 연구의 세그먼트에는 샘플 및 유형 영역이 포함됩니다.
- 제약 무균 시험 산업에 대한 규제 프레임워크를 정의하고 분석합니다.
- 다양한 중개인의 존재와 관련된 가치 사슬을 분석하고 산업의 고객 및 경쟁사 행동을 분석합니다.
- 주요 지역에 대한 제약 무균 시험 시장의 현재 및 예측 시장 규모를 분석합니다.
- 보고서에서 연구된 지역의 주요 국가에는 아시아 태평양, 유럽, 북미 및 기타 지역이 포함됩니다.
- 빠르게 성장하는 시장에서 지속하기 위해 시장 참여자가 채택한 성장 전략과 함께 제약 무균 시험 시장의 회사 프로필
- 산업에 대한 심층적인 지역 수준 분석
관련 보고서
이 상품을 구매한 고객님들도 함께 구매하신 상품