- Strona główna
- O nas
- Branża
- Usługi
- Czytanie
- Kontakt
Rynek terapii genowej opartej na wirusach adeno-asocjowanych: bieżąca analiza i prognoza (2024-2032)
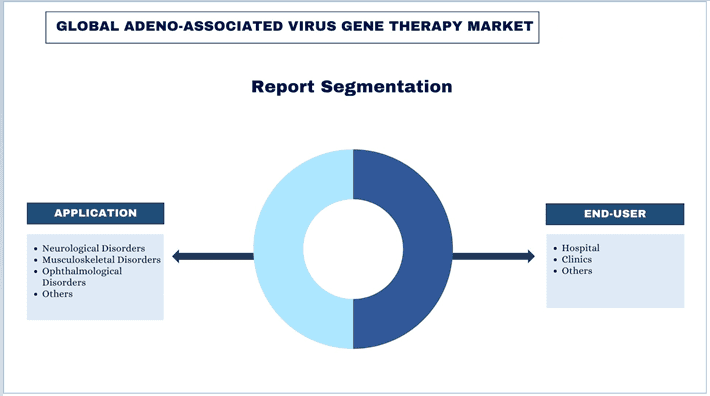
Nacisk na Zastosowanie (Zaburzenia Neurologiczne, Zaburzenia Mięśniowo-Szkieletowe, Zaburzenia Okulistyczne i Inne); Użytkownik Końcowy (Szpital, Kliniki i Inne); Region/Kraj.
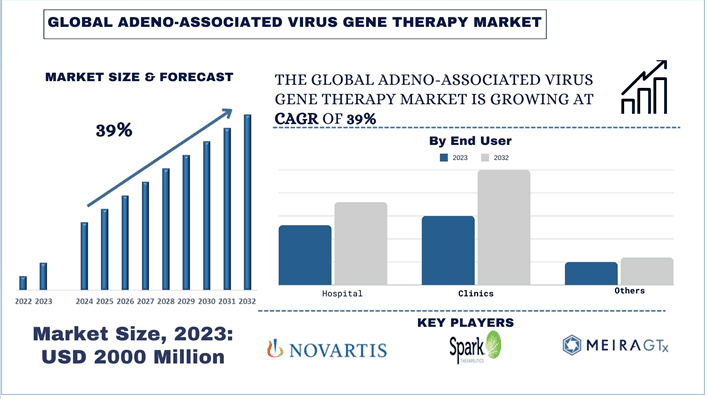
Wielkość i prognozy rynku terapii genowej z użyciem wirusów AAV
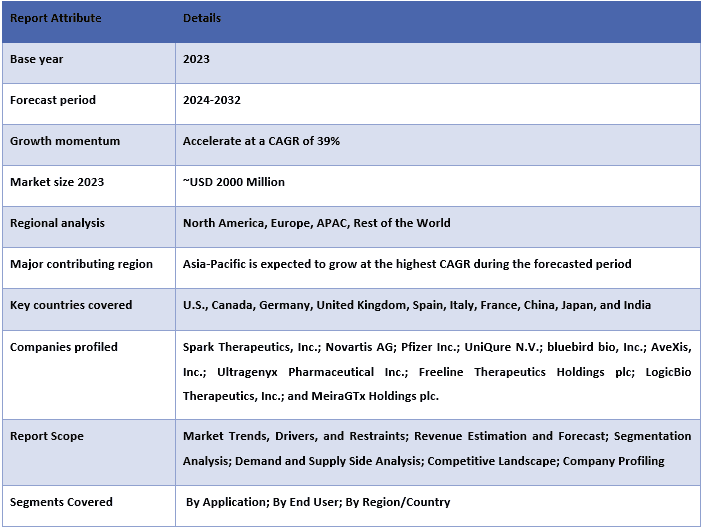
Wartość rynku terapii genowej z użyciem wirusów AAV szacowano na około ~2000 milionów USD w 2023 roku, a oczekuje się, że w okresie prognozy (2024-2032) wzrośnie on przy silnym CAGR wynoszącym około 39%, ze względu na rosnący popyt na terapię celowaną.
Analiza rynku terapii genowej z użyciem wirusów AAV
Odkrycie i zrozumienie nowych wektorów dostarczania genów przyczyniło się do niedawnego wzrostu inicjatyw w zakresie terapii genowej. Wirus związany z adenowirusami (AAV) to wirus bezotoczkowy, który wzbudził duże zainteresowanie w branży, szczególnie w eksperymentalnych technikach terapeutycznych w fazie klinicznej. AAV można modyfikować, aby przenosił DNA do komórek docelowych. Jedną z najbezpieczniejszych metod terapii genowych do tej pory jest zdolność do wytwarzania rekombinowanych cząstek AAV z sekwencjami DNA będącymi przedmiotem zainteresowania dla różnych zastosowań terapeutycznych, ale bez żadnych genów wirusowych.
Trendy na rynku terapii genowej z użyciem wirusów AAV
W tej sekcji omówiono kluczowe trendy rynkowe, które wpływają na różne segmenty rynku terapii genowej z użyciem wirusów AAV, zidentyfikowane przez nasz zespół ekspertów ds. badań.
Segment zaburzeń neurologicznych przekształca branżę
Zaburzenia neurologiczne zyskują znaczną uwagę na rynku terapii genowej AAV (wirus związany z adenowirusami) ze względu na kilka istotnych czynników. Po pierwsze, obciążenie chorobami neurologicznymi, takimi jak choroba Alzheimera, Parkinsona i różne schorzenia genetyczne, rośnie na całym świecie, co napędza popyt na innowacyjne metody leczenia. Tradycyjne terapie często nie radzą sobie skutecznie z zarządzaniem tymi złożonymi stanami, co stwarza pilną potrzebę zaawansowanych rozwiązań. Terapia genowa AAV oferuje obiecujące podejście, dostarczając precyzyjne modyfikacje genetyczne bezpośrednio do dotkniętych komórek, potencjalnie rozwiązując pierwotne przyczyny tych zaburzeń. Ponadto postępy w technologii AAV poprawiły jej profile bezpieczeństwa i skuteczności, czyniąc ją bardziej opłacalną opcją do celowania w centralny układ nerwowy. Rosnące wsparcie agencji regulacyjnych dla badań nad terapią genową i rosnące inwestycje w badania nad neuronauką dodatkowo wspierają rozwój terapii opartych na AAV w leczeniu zaburzeń neurologicznych. Czynniki te łącznie przyczyniają się do zwiększonego zainteresowania i szybkiego rozwoju terapii genowej AAV w dziedzinie neurologii.
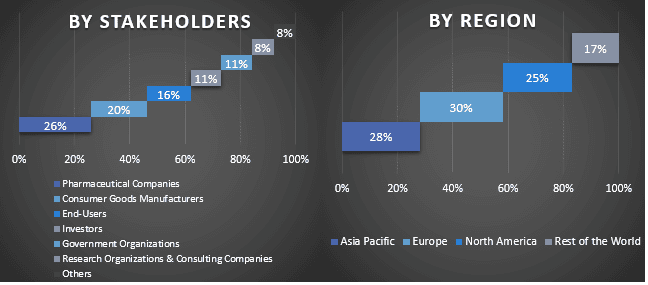
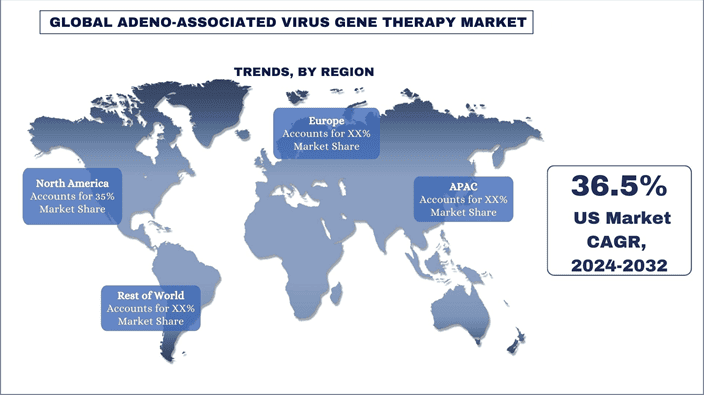
Oczekuje się, że Ameryka Północna będzie rosła ze znacznym CAGR w okresie prognozy
W Ameryce Północnej Stany Zjednoczone mają duży udział w rynku. Obecność firm farmaceutycznych w regionie, wspierające programy rządowe i wzrost inwestycji w badania i rozwój napędzają wzrost rynku w nadchodzących latach. Na przykład w kwietniu 2024 roku amerykańska Agencja ds. Żywności i Leków (FDA) zatwierdziła lek Pfizer BEQVEZ (fidanacogene elaparvovec-dzkt), jednorazową terapię genową dla dorosłych z hemofilią B. Ponadto wzrost częstości występowania zaburzeń neurologicznych i mięśniowo-szkieletowych również zwiększa popyt na terapię genową AAV w regionie. Według Fundacji Parkinsona, w 2022 roku w USA u prawie 90 000 osób diagnozuje się chorobę Parkinsona każdego roku. Stanowi to gwałtowny wzrost o 50% w stosunku do wcześniej szacowanej liczby 60 000 diagnoz rocznie.
Przegląd branży terapii genowej z użyciem wirusów AAV
Rynek terapii genowej z użyciem wirusów AAV jest konkurencyjny, z kilkoma globalnymi i międzynarodowymi graczami rynkowymi. Kluczowi gracze przyjmują różne strategie rozwoju, aby zwiększyć swoją obecność na rynku, takie jak partnerstwa, umowy, współpraca, wprowadzanie nowych typów, ekspansja geograficzna oraz fuzje i przejęcia. Niektóre z głównych graczy działających na rynku to: Spark Therapeutics, Inc.; Novartis AG; Pfizer Inc.; UniQure N.V.; bluebird bio, Inc.; AveXis, Inc.; Ultragenyx Pharmaceutical Inc.; Freeline Therapeutics Holdings plc; LogicBio Therapeutics, Inc.; i MeiraGTx Holdings plc.
Wiadomości z rynku terapii genowej z użyciem wirusów AAV
W styczniu 2024 r. Charles River Laboratories wprowadził na rynek gotową ofertę plazmidów Rep/Cap, zaprojektowaną w celu usprawnienia programów terapii genowej opartej na wirusach AAV.
W czerwcu 2023 r. Amerykańska FDA przyznała zatwierdzenie lekowi Roctavian, terapii genowej opartej na wektorze wirusa AAV do leczenia dorosłych z ciężką hemofilią A bez wcześniej istniejących przeciwciał przeciwko serotypowi 5 wirusa AAV, wykrytych za pomocą testu zatwierdzonego przez FDA.
Zakres raportu dotyczącego rynku terapii genowej z użyciem wirusów AAV
Powody zakupu tego raportu:
- Badanie obejmuje analizę wielkości rynku i prognozowania, zatwierdzoną przez uwierzytelnionych kluczowych ekspertów branżowych.
- Raport przedstawia szybki przegląd ogólnej wydajności branży na pierwszy rzut oka.
- Raport obejmuje dogłębną analizę czołowych podmiotów z branży, z głównym naciskiem na kluczowe dane finansowe, portfele typów, strategie ekspansji i najnowsze wydarzenia.
- Szczegółowe badanie czynników napędzających, ograniczeń, kluczowych trendów i możliwości występujących w branży.
- Badanie kompleksowo obejmuje rynek w różnych segmentach.
- Dogłębna analiza branży na poziomie regionalnym.
Opcje dostosowywania:
Globalny rynek terapii genowej z użyciem wirusów AAV można dodatkowo dostosować zgodnie z wymaganiami lub dowolnym innym segmentem rynku. Poza tym UMI rozumie, że możesz mieć własne potrzeby biznesowe, dlatego skontaktuj się z nami, aby otrzymać raport, który w pełni odpowiada Twoim wymaganiom.
Spis treści
Metodologia badań dla analizy rynku terapii genowej opartej na wirusach AAV (2024-2032)
Analiza historycznego rynku, szacowanie obecnego rynku i prognozowanie przyszłego rynku globalnej terapii genowej opartej na wirusach AAV to trzy główne kroki podjęte w celu stworzenia i analizy stopnia wdrożenia terapii genowej opartej na wirusach AAV w głównych regionach na świecie. Przeprowadzono wyczerpujące badania wtórne w celu zebrania historycznych danych rynkowych i oszacowania obecnej wielkości rynku. Po drugie, w celu potwierdzenia tych spostrzeżeń wzięto pod uwagę liczne ustalenia i założenia. Ponadto przeprowadzono również wyczerpujące wywiady pierwotne z ekspertami branżowymi w całym łańcuchu wartości globalnego rynku terapii genowej opartej na wirusach AAV. Po założeniu i walidacji danych rynkowych poprzez wywiady pierwotne, zastosowaliśmy podejście odgórne/oddolne do prognozowania całkowitej wielkości rynku. Następnie przyjęto metody podziału rynku i triangulacji danych w celu oszacowania i analizy wielkości rynku segmentów i podsegmentów, których dotyczy branża. Szczegółowa metodologia została opisana poniżej:
Analiza historycznej wielkości rynku
Krok 1: Dogłębne badanie źródeł wtórnych:
Przeprowadzono szczegółowe badanie wtórne w celu uzyskania historycznej wielkości rynku terapii genowej opartej na wirusach AAV poprzez wewnętrzne źródła firm, takie jak raporty roczne i sprawozdania finansowe, prezentacje wyników, komunikaty prasowe itp., oraz źródła zewnętrzne, w tym czasopisma, wiadomości i artykuły, publikacje rządowe, publikacje konkurencji, raporty sektorowe, bazy danych stron trzecich i inne wiarygodne publikacje.
Krok 2: Segmentacja rynku:
Po uzyskaniu historycznej wielkości rynku terapii genowej opartej na wirusach AAV przeprowadziliśmy szczegółową analizę wtórną w celu zebrania historycznych informacji o rynku i udziałach dla różnych segmentów i podsegmentów w głównych regionach. Główne segmenty zawarte w raporcie to zastosowanie, użytkownik końcowy i regiony. Ponadto przeprowadzono analizy na poziomie krajów w celu oceny ogólnego wdrożenia modeli testowania w danym regionie.
Krok 3: Analiza czynnikowa:
Po uzyskaniu historycznej wielkości rynku dla różnych segmentów i podsegmentów przeprowadziliśmy szczegółową analizę czynnikową w celu oszacowania obecnej wielkości rynku terapii genowej opartej na wirusach AAV. Ponadto przeprowadziliśmy analizę czynnikową przy użyciu zmiennych zależnych i niezależnych, takich jak zastosowanie, użytkownik końcowy i regiony rynku terapii genowej opartej na wirusach AAV. Przeprowadzono dogłębną analizę scenariuszy popytu i podaży, biorąc pod uwagę najważniejsze partnerstwa, fuzje i przejęcia, ekspansję działalności i wprowadzenie typów na rynek w sektorze terapii genowej opartej na wirusach AAV na całym świecie.
Szacowanie i prognozowanie obecnej wielkości rynku
Określenie obecnej wielkości rynku: W oparciu o praktyczne spostrzeżenia z powyższych 3 kroków ustaliliśmy obecną wielkość rynku, kluczowych graczy na globalnym rynku terapii genowej opartej na wirusach AAV oraz udziały w rynku poszczególnych segmentów. Wszystkie wymagane udziały procentowe, podziały i rozkłady rynkowe zostały określone przy użyciu wspomnianego powyżej podejścia wtórnego i zweryfikowane poprzez wywiady pierwotne.
Szacowanie i prognozowanie: Do szacowania i prognozowania rynku przypisano wagi różnym czynnikom, w tym czynnikom napędzającym i trendom, ograniczeniom i możliwościom dostępnym dla interesariuszy. Po przeanalizowaniu tych czynników zastosowano odpowiednie techniki prognozowania, tj. podejście odgórne/oddolne, aby dojść do prognozy rynkowej na rok 22032 dla różnych segmentów i podsegmentów na głównych rynkach na świecie. Metodologia badań przyjęta do oszacowania wielkości rynku obejmuje:
- Wielkość rynku branży pod względem przychodów (USD) i wskaźnika wdrożenia terapii genowej opartej na wirusach AAV na głównych rynkach krajowych
- Wszystkie udziały procentowe, podziały i rozkłady segmentów i podsegmentów rynku
- Kluczowi gracze na globalnym rynku terapii genowej opartej na wirusach AAV pod względem oferowanych typów. Ponadto strategie rozwoju przyjęte przez tych graczy, aby konkurować na szybko rozwijającym się rynku
Walidacja wielkości rynku i udziału
Badania pierwotne: Przeprowadzono dogłębne wywiady z kluczowymi liderami opinii (KOL), w tym kadrą kierowniczą najwyższego szczebla (CXO/wiceprezesi, szef sprzedaży, szef marketingu, szef operacyjny, szef regionalny, szef krajowy itp.) w głównych regionach. Następnie podsumowano wyniki badań pierwotnych i przeprowadzono analizę statystyczną w celu udowodnienia postawionej hipotezy. Dane wejściowe z badań pierwotnych zostały połączone z ustaleniami wtórnymi, przekształcając w ten sposób informacje w praktyczne spostrzeżenia.
Podział uczestników pierwotnych w różnych regionach
Inżynieria rynku
Zastosowano technikę triangulacji danych, aby ukończyć ogólne szacowanie rynku i uzyskać dokładne dane statystyczne dla każdego segmentu i podsegmentu globalnego rynku terapii genowej opartej na wirusach AAV. Dane zostały podzielone na kilka segmentów i podsegmentów po przestudiowaniu różnych parametrów i trendów w obszarach zastosowania, użytkownika końcowego i regionów na globalnym rynku terapii genowej opartej na wirusach AAV.
Główny cel globalnego badania rynku terapii genowej opartej na wirusach AAV
W badaniu określono aktualne i przyszłe trendy rynkowe globalnego rynku terapii genowej opartej na wirusach AAV. Inwestorzy mogą uzyskać strategiczne spostrzeżenia, na których mogą oprzeć swoje decyzje dotyczące inwestycji, na podstawie analizy jakościowej i ilościowej przeprowadzonej w badaniu. Aktualne i przyszłe trendy rynkowe określiły ogólną atrakcyjność rynku na poziomie regionalnym, zapewniając platformę dla uczestników przemysłu do wykorzystania niewykorzystanego rynku, aby skorzystać z przewagi pioniera. Inne cele ilościowe badań obejmują:
- Analiza aktualnej i prognozowanej wielkości rynku terapii genowej opartej na wirusach AAV pod względem wartości (USD). Ponadto analiza aktualnej i prognozowanej wielkości rynku dla różnych segmentów i podsegmentów.
- Segmenty w badaniu obejmują obszary zastosowania, użytkownika końcowego i regiony.
- Zdefiniowanie i analiza ram regulacyjnych dla terapii genowej opartej na wirusach AAV
- Analiza łańcucha wartości związanego z obecnością różnych pośredników, wraz z analizą zachowań klientów i konkurentów w branży.
- Analiza aktualnej i prognozowanej wielkości rynku terapii genowej opartej na wirusach AAV dla głównego regionu.
- Główne kraje regionów badanych w raporcie to Azja i Pacyfik, Europa, Ameryka Północna i Reszta Świata
- Profile firm z rynku terapii genowej opartej na wirusach AAV oraz strategie rozwoju przyjęte przez uczestników rynku w celu utrzymania się na szybko rozwijającym się rynku.
- Dogłębna analiza branży na poziomie regionalnym.
Najczęściej zadawane pytania FAQ
Pytanie 1: Jaka jest obecna wielkość rynku terapii genowej opartej na wirusach adeno-asocjowanych (AAV) i jej potencjał wzrostu?
P2: Jakie czynniki napędzają wzrost rynku terapii genowej opartej na wirusach adeno-asocjowanych?
P3: Który segment ma największy udział w rynku terapii genowej opartej na wirusach adeno-asocjowanych ze względu na użytkownika końcowego?
P4: Jakie są nowe technologie i trendy na rynku terapii genowej opartej na wirusach adeno-asocjowanych?
P5: Który region zdominuje rynek terapii genowej z wykorzystaniem wirusa adeno-asocjowanego?
Powiązane Raporty
Klienci, którzy kupili ten przedmiot, kupili również