- Strona główna
- O nas
- Branża
- Usługi
- Czytanie
- Kontakt
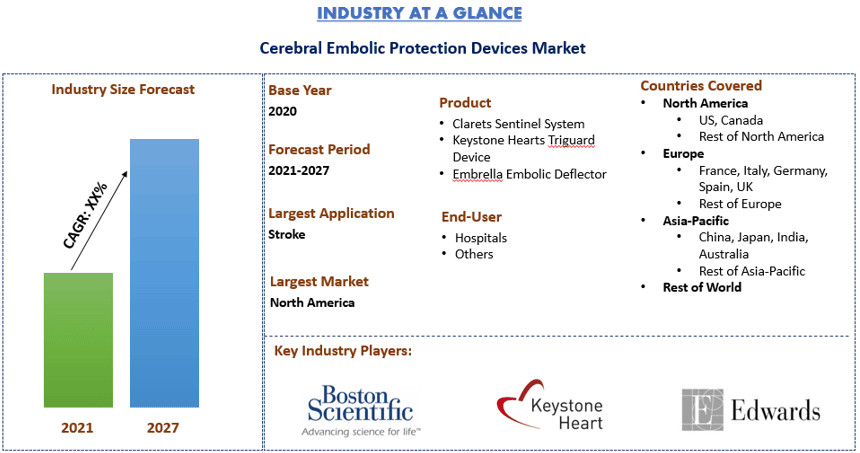
Rynek Urządzeń do Ochrony Przed Zatorami Mózgowymi: Aktualna Analiza i Prognoza (2021-2027)
Nacisk na Produkt (System Clarets Sentinel, Urządzenie Keystone Hearts Triguard i Deflektor Zatorów Embrella); Użytkownicy Końcowi (Szpitale i Inne); oraz Region i Kraj.
MózgowyWartość rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi oszacowano na ~650 milionów USD w 2020 roku i oczekuje się, że będzie rósł w tempie CAGR ~8% w okresie prognozy (2021-2027).Jednym z najbardziej obawianych powikłań po przezskórnym wszczepieniu zastawki aortalnej jest udar mózgu lub przemijający atak niedokrwienny. Urządzenia do ochrony przed zatorami mózgowymi zmniejszają ryzyko incydentów naczyniowo-mózgowych po TAVR. Urządzenia te mają na celu zapobieganie przedostawaniu się resztek proceduralnych do krążenia mózgowego. Urządzenia do ochrony przed zatorami mózgowymi (CEPDs) zostały opracowane w celu zmniejszenia ryzyka incydentów naczyniowo-mózgowych i cichych zatorów. Są skuteczne w filtrowaniu resztek i zwiększaniu objętości zmian niedokrwiennych zatorowych, zmniejszając w ten sposób ryzyko incydentów naczyniowo-mózgowych. CEPDs to filtry siatkowe zapobiegające przedostawaniu się materiału zatorowego do tętnic szyjnych. Istnieje wiele rodzajów CEPDs, różniących się wielkością porów, miejscem wdrożenia i składem chemicznym.Według badania, wskaźnik udanego wdrożenia osiągany jest w ponad 90% przypadków, a wskaźniki sukcesu wahają się od 64-100%.CEPDs mogą wychwytywać materiały takie jak zakrzepy, tkanka zastawkowa, tkanka zastawki tętniczej, materiały obce i zwapnienia.
Kluczowymi czynnikami wpływającymi na wzrost urządzeń do ochrony przed zatorami mózgowymi są postępy technologiczne w opiece zdrowotnej, a także, ze względu na rosnącą starzejącą się populację, rośnie liczba osób potrzebujących procedur TAVR.Oczekuje się, że amerykańska populacja w grupie wiekowej 65 lat i starszej podwoi się w latach 2018-2060. Przewiduje się, że populacja wzrośnie z 52 milionów osób w 2018 r. do 95 milionów osób w 2060 r., co oznacza, że całkowity udział populacji przesunie się z 16% do 23%.Wszystkie te czynniki wpływają na wzrost rynku urządzeń do ochrony przed zatorami mózgowymi w okresie prognozy.
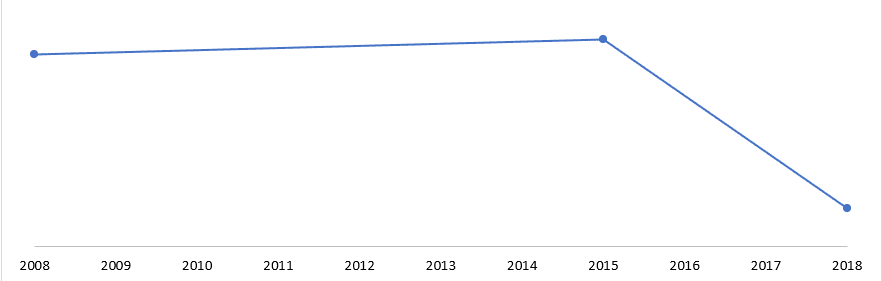
Wskaźniki śmiertelności w Stanach Zjednoczonych z powodu zwężenia zastawki aortalnej na przestrzeni lat (%), 2008-2018
Boston Scientific, Keystone Heart i Edward Lifesciences to niektórzy z czołowych graczy działających na rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi. Kilka fuzji i przejęć wraz z partnerstwami zostało podjętych przez tych graczy w celu zapewnienia klientom zaawansowanych technologicznie i innowacyjnych produktów/technologii.
Wgląd zaprezentowany w raporcie
„Wśród produktów, segment Urządzenia Keystone Heart’s Triguard ma największy udział”
W oparciu o produkt, urządzenia do ochrony przed zatorami mózgowymi są podzielone na System Clarets Sentinel, Urządzenie Keystones Heart’s Triguard i Deflektor Embrella Embolic. Segment urządzenia trigaurd serca na rynku urządzeń do ochrony przed zatorami mózgowymi został wyceniony na XX milionów USD w 2020 roku i prawdopodobnie osiągnie XX milionów USD do 2027 roku, rosnąc w tempie CAGR XX% w latach 2021-2027. Triguar to jedyny system zatwierdzony znakiem CE. Może pokryć i chronić wszystkie trzy główne mózgowe naczynia aorty. System ochrony mózgowej Sentinel ma dwa filtry wewnątrz cewnika dostarczającego 6F. Pomimo wydłużonego czasu fluoroskopii,wdrożenie zwykle trwa mniej niż 10 minut w 91% przypadków. Sentinel może chronić 74% objętości mózgu, podczas gdy embralla i triguard mogą chronić odpowiednio 74% i 100%.Szacuje się, że rynek ochrony mózgowej będzie rósł wraz ze wzrostem liczby przypadków dysfunkcji zastawek na świecie, a także wraz z większą liczbą urządzeń uzyskujących aprobatę FDA.
„Wśród użytkowników końcowych, segment Szpitale ma największy udział”
W oparciu o użytkownika końcowego, rynek jest podzielony na szpitale i inne. Segment szpitali wygenerował przychody w wysokości XX milionów USD w 2020 roku i oczekuje się, że wzrośnie w tempie CAGR XX% w okresie prognozy, aby osiągnąć wycenę rynkową w wysokości XX milionów USD do 2027F. Urządzenia do ochrony przed zatorami mózgowymi są używane podczas procedur TAVR, które są wykonywane u pacjentów ze zwężeniem zastawki aortalnej i nie mogą przejść operacji na otwartym sercu.Urządzenia do ochrony przed zatorami mózgowymi mogą zmniejszyć ryzyko udarów, zbierając do 90-95% resztek.
„Ameryka Północna reprezentuje jeden z największych rynków Urządzeń do Ochrony Przed Zatorami Mózgowymi”
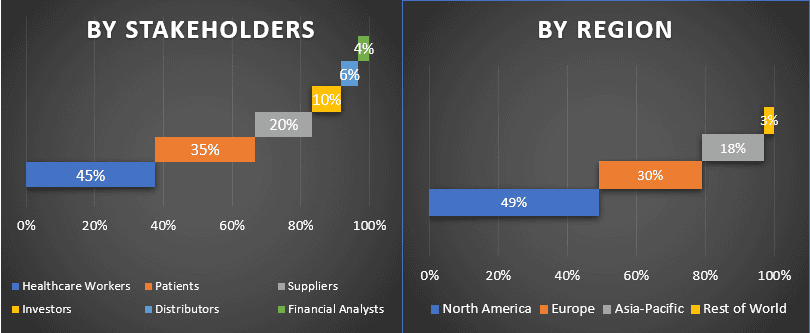
Aby lepiej zrozumieć dynamikę rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi, przeprowadzono szczegółową analizę dla różnych regionów na całym świecie, w tym Ameryki Północnej (USA, Kanady i reszty Ameryki Północnej), Europy (Niemcy, Francja, Włochy, Wielka Brytania, Hiszpania i reszta Europy), Azji i Pacyfiku (Chiny, Japonia, Indie, Australia i reszta APAC) i reszty świata. Ameryka Północna zdominowała rynek i zdobyła około XX% udziału w rynku, ze względu na wzrost populacji geriatrycznej i występowanie zwężenia aorty.
Powody, dla których warto kupić ten raport:
- Badanie obejmuje analizę wielkości rynku i prognozowania zatwierdzoną przez uwierzytelnionych kluczowych ekspertów branżowych
- Raport przedstawia szybki przegląd ogólnej wydajności branży na pierwszy rzut oka
- Raport zawiera dogłębną analizę wybitnych podmiotów z branży, ze szczególnym uwzględnieniem kluczowych danych finansowych przedsiębiorstwa, portfolio produktów, strategii ekspansji i ostatnich wydarzeń
- Szczegółowe badanie czynników napędzających, ograniczeń, kluczowych trendów i możliwości występujących w branży
- Badanie kompleksowo obejmuje rynek w różnych segmentach
- Dogłębna analiza na poziomie regionalnym branży
Opcje dostosowywania:
Rynek Urządzeń do Ochrony Przed Zatorami Mózgowymi można dalej dostosować zgodnie z wymaganiami lub innym segmentem rynku. Poza tym UMI rozumie, że możesz mieć własne potrzeby biznesowe, dlatego zapraszamy do kontaktu, aby otrzymać raport, który w pełni odpowiada Twoim wymaganiom.
Spis treści
Analiza historycznego rynku, oszacowanie bieżącego rynku i prognozowanie przyszłego rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi były trzema głównymi krokami podjętymi w celu stworzenia i przeanalizowania wdrożenia Urządzeń do Ochrony Przed Zatorami Mózgowymi w głównych regionach na świecie. Przeprowadzono wyczerpujące badania wtórne w celu zebrania historycznych danych rynkowych i oszacowania obecnej wielkości rynku. Po drugie, aby zweryfikować te spostrzeżenia, wzięto pod uwagę liczne ustalenia i założenia. Ponadto przeprowadzono wyczerpujące wywiady pierwotne z ekspertami branżowymi z całego łańcucha wartości rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi. Po założeniu i weryfikacji danych rynkowych poprzez wywiady pierwotne, zastosowaliśmy podejście top-down/bottom-up do prognozowania całkowitej wielkości rynku. Następnie zastosowano metody podziału rynku i triangulacji danych w celu oszacowania i analizy wielkości rynku segmentów i podsegmentów, do których należy branża. Szczegółowa metodologia wyjaśniona poniżej:
Analiza historycznej wielkości rynku
Krok 1: Dogłębne badanie źródeł wtórnych:
Szczegółowe badanie wtórne zostało przeprowadzone w celu uzyskania historycznej wielkości rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi poprzez wewnętrzne źródła firmy, takie jakraport roczny i sprawozdania finansowe, prezentacje wyników, komunikaty prasowe itp.,oraz źródła zewnętrzne, w tymczasopisma, wiadomości i artykuły, publikacje rządowe, publikacje konkurencji, raporty sektorowe, bazy danych stron trzecich i inne wiarygodne publikacje.
Krok 2: Segmentacja rynku:
Po uzyskaniu historycznej wielkości rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi przeprowadziliśmy szczegółową analizę wtórną w celu zebrania historycznych informacji rynkowych i udziałów dla różnych segmentów i podsegmentów dla głównych regionów. Główne segmenty zawarte w raporcie to produkt i użytkownik końcowy. Ponadto przeprowadzono analizy na poziomie kraju w celu oceny ogólnego wdrożenia Urządzeń do Ochrony Przed Zatorami Mózgowymi w tym regionie.
Krok 3: Analiza czynników:
Po uzyskaniu historycznej wielkości rynku różnych segmentów i podsegmentów przeprowadziliśmy szczegółowąanalizę czynnikóww celu oszacowania obecnej wielkości rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi. Ponadto przeprowadziliśmy analizę czynników przy użyciu zmiennych zależnych i niezależnych, takich jak wzrost populacji geriatrycznej i świadomości wśród ludzi. Przeprowadzono dokładną analizę scenariuszy popytu i podaży, biorąc pod uwagę najlepsze partnerstwa, fuzje i przejęcia, ekspansję biznesową i wprowadzenie produktów w sektorze Urządzeń do Ochrony Przed Zatorami Mózgowymi na całym świecie.
Oszacowanie i prognoza aktualnej wielkości rynku
Określanie bieżącej wielkości rynku:W oparciu o przydatne spostrzeżenia z powyższych 3 kroków, ustaliliśmy aktualną wielkość rynku, kluczowych graczy na rynku Urządzeń do Ochrony Przed Zatorami Mózgowymi oraz udziały rynkowe segmentów. Wszystkie wymagane udziały procentowe, podziały i podziały rynku zostały określone za pomocą wyżej wymienionego podejścia wtórnego i zostały zweryfikowane poprzez wywiady pierwotne.
Szacowanie i prognozowanie:Do oszacowania i prognozy rynku przypisano wagi do różnych czynników, w tym czynników napędzających i trendów, ograniczeń i możliwości dostępnych dla interesariuszy. Po przeanalizowaniu tych czynników zastosowano odpowiednie techniki prognozowania, tj. podejście bottom-up/top-down, aby uzyskać prognozę rynkową do 2027 roku dla różnych segmentów i podsegmentów na głównych rynkach na świecie. Metodologia badawcza przyjęta do oszacowania wielkości rynku obejmuje:
- Wielkość rynku branży, pod względem wartości (USD) oraz wskaźnik adopcji urządzeń do ochrony przed zatorami mózgowymi na głównych rynkach krajowych
- Wszystkie udziały procentowe, podziały i podziały segmentów i podsegmentów rynku
- Kluczowi gracze na rynku urządzeń do ochrony przed zatorami mózgowymi pod względem oferowanych produktów. Ponadto strategie wzrostu przyjęte przez tych graczy w celu konkurowania na szybko rozwijającym się rynku
Weryfikacja wielkości i udziału w rynku
Badania pierwotne:Przeprowadzono dogłębne wywiady z kluczowymi liderami opinii (KOL), w tym z kadrą kierowniczą wyższego szczebla (CXO/Wiceprezesi, szefowie sprzedaży, szefowie marketingu, szefowie operacyjni i regionalni, szefowie krajowi itp.) w głównych regionach. Wyniki badań pierwotnych zostały następnie podsumowane i przeprowadzono analizę statystyczną w celu udowodnienia postawionej hipotezy. Dane z badań pierwotnych zostały skonsolidowane z ustaleniami wtórnymi, przekształcając tym samym informacje w przydatne wnioski.
Podział uczestników pierwotnych w różnych regionach
Inżynieria rynku
Zastosowano technikę triangulacji danych w celu uzupełnienia ogólnych szacunków rynku i uzyskania precyzyjnych danych statystycznych dla każdego segmentu i podsegmentu rynku urządzeń do ochrony przed zatorami mózgowymi. Dane podzielono na kilka segmentów i podsegmentów po przestudiowaniu różnych parametrów i trendów w obszarach typu i ich rodzaju rynku urządzeń do ochrony przed zatorami mózgowymi.
Główny cel badania rynku urządzeń do ochrony przed zatorami mózgowymi
W badaniu wskazano obecne i przyszłe trendy rynkowe urządzeń do ochrony przed zatorami mózgowymi. Inwestorzy mogą uzyskać strategiczne spostrzeżenia, aby oprzeć swoje decyzje inwestycyjne na analizie jakościowej i ilościowej przeprowadzonej w badaniu. Obecne i przyszłe trendy rynkowe określono ogólną atrakcyjność rynku na poziomie regionalnym, zapewniając platformę uczestnikom branżowym do wykorzystania niewykorzystanego rynku i czerpania korzyści jako pierwszego gracza. Inne cele ilościowe badań obejmują:
- Analiza bieżącej i prognozowanej wielkości rynku urządzeń do ochrony przed zatorami mózgowymi pod względem wartości (USD). Ponadto analiza bieżącej i prognozowanej wielkości rynku różnych segmentów i podsegmentów
- Segmenty w badaniu obejmują obszary typu i ich podtypy
- Definicja i analiza ram regulacyjnych dla branży urządzeń do ochrony przed zatorami mózgowymi
- Analiza łańcucha wartości związana z obecnością różnych pośredników, wraz z analizą zachowań klientów i konkurentów w branży
- Analiza bieżącej i prognozowanej wielkości rynku urządzeń do ochrony przed zatorami mózgowymi dla głównych regionów
- Główne regiony objęte badaniem obejmują Amerykę Północną (USA, Kanada i reszta Ameryki Północnej), Europę (Niemcy, Wielka Brytania, Francja, Hiszpania, Włochy i reszta Europy), Azję i Pacyfik (Chiny, Japonia, Indie, Australia i reszta Azji i Pacyfiku) oraz Resztę Świata
- Profile firm na rynku urządzeń do ochrony przed zatorami mózgowymi oraz strategie wzrostu przyjęte przez graczy rynkowych w celu utrzymania się na szybko rozwijającym się rynku
- Dogłębna analiza branży na poziomie regionalnym
Powiązane Raporty
Klienci, którzy kupili ten przedmiot, kupili również