- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
Virtual Clinical Trials Market: Current Analysis and Forecast (2023-2030)
Emphasis on Study Design (Interventional, Observational, Expanded Access); Therapeutic Area (CNS, Autoimmune/Inflammation, Cardiovascular Disease, Metabolic/Endocrinology, Infectious Disease, Oncology, Others); Phase (Phase I, Phase II, Phase III, Phase IV); and Region/Country
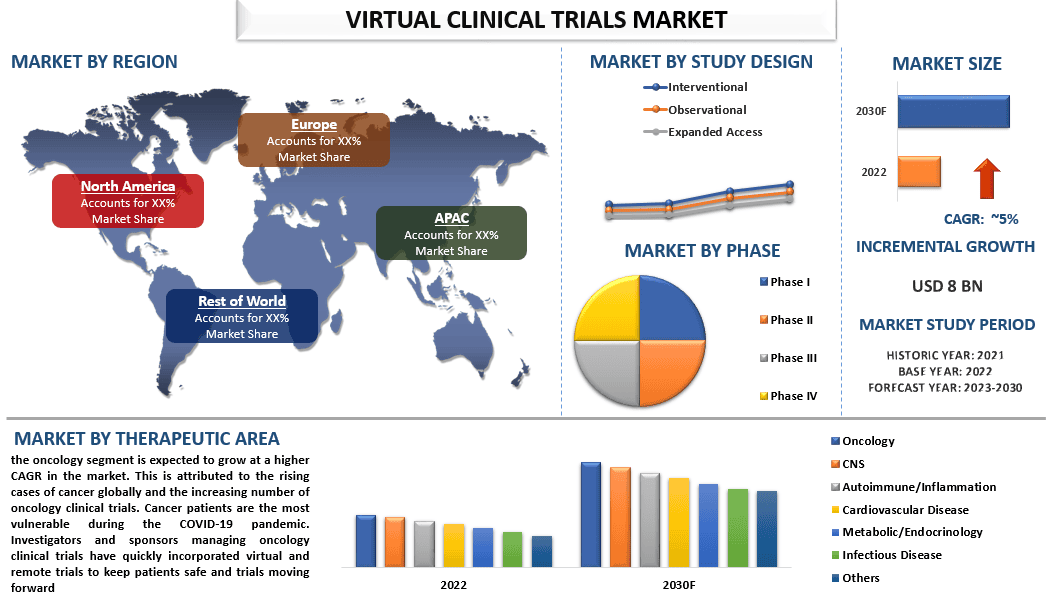
The Virtual clinical trials market is valued at USD 8 billion in 2022 & is expected to grow at a CAGR of 5% from 2023-2030. Virtual clinical trials (also known as decentralized clinical trials or remote clinical trials) are a modern approach to conducting clinical research and drug development that utilizes digital technologies and remote methods to gather data from participants. In traditional clinical trials, participants typically need to visit physical research centers or hospitals for assessments and data collection. In contrast, virtual clinical trials leverage digital tools, such as mobile health apps, wearable devices, telemedicine, and electronic data capture systems, to enable remote data collection and monitoring. The growing demand for Virtual Clinical Trials can be attributed to patient-centric approaches around the globe. Virtual clinical trials offer increased convenience and flexibility for participants, as they can remotely contribute data from their homes or local healthcare facilities. This patient-centric approach helps improve patient recruitment and retention rates, leading to faster and more diverse participant enrollment. These approaches boost the demand of the market and attract companies to collaborate and invest in the market. For Instance, in 2022, Oracle and ObvioHealth entered a strategic collaboration to integrate diverse data sets into virtual/decentralized clinical trials in the Asia Pacific region. This initiative is expected to allow the quick collection, integration and analysis of multi-source data collected from labs, devices, patients, and sites. Furthermore, the blood grouping reagent market has witnessed rapid advancements in digital health technologies that have made virtual clinical trials more feasible and accessible. The availability of smartphones, wearable devices, remote monitoring tools, and telemedicine platforms has enabled remote data collection, real-time monitoring, and virtual interactions between researchers and participants. In addition, Virtual clinical trials allow for continuous data collection and real-time monitoring of participants, leading to more comprehensive and accurate data. Wearable devices and mobile health apps enable the collection of objective and real-world data, providing researchers with valuable insights into participants’ daily lives and health conditions. However, some of the restraints in the market, including the high costs of advanced reagents and stringent regulations are impeding the growth of this market all over the world.
Some of the major players operating in the market include ICON, plc; Parexel International Corporation; IQVIA; Covance; Medable, Inc.; Signant Health; Oracle; Halo Health Systems; Croprime; LEO Innovation Lab. Several M&As along with partnerships have been undertaken by these players to facilitate customers with hi-tech and innovative products/technologies.
Insights Presented in the Report
“Amongst study design, the interventional segment held a significant share of the market in 2022.”
Based on the study design, the market has been categorized into interventional, observational, and expanded access. Among them, the interventional segment is expected to grow at the highest CAGR in the market. The rapid increase in the number of experiments to develop novel medications for various diseases and the digitalization of laboratories are factors driving the segment. The outbreak of coronavirus has raised the demand for testing and trials of new drugs and vaccines to combat the situation around the world as the traditional method of clinical trials comes with a huge risk of infection in people.
“Amongst phase, the Phase II segment held the higher CAGR in the market in 2022.”
Based on the phase, the market has been categorized into Phase I, Phase II, Phase III, and Phase IV. Among them, the Phase II category is to witness higher adoption of virtual clinical trials during the forecast period. This is largely due to the adoption of DCT tools and platforms in phase II and phase I clinical trial procedures for patient participation. Virtual clinical trials are most beneficial in phase II of the clinical trials as It delivers value to the biopharmaceutical and pharmaceutical industry by saving time-sensitive patient data, delayed approvals, and site payments.
“North America dominated the Virtual clinical trials market in 2022.”
Virtual Clinical Trials have gained increasing popularity in North America due to various factors. Regulatory agencies in North America, such as the U.S. Food and Drug Administration (FDA) and Health Canada, have shown support for virtual clinical trials. They have issued guidelines and provided flexibility to adapt to remote trial methodologies, facilitating the growth of virtual trials in the region. For instance, In February 2021, the FDA released new guidance on conducting clinical trials during the COVID-19 pandemic, emphasizing the use of remote and decentralized approaches, including virtual trials, to ensure patient safety and trial continuity. Moreover, North America is home to many technology companies and innovative startups that are driving advancements in digital health technologies. The availability of sophisticated platforms, wearable devices, telemedicine solutions, and data analytics tools facilitates the implementation of virtual clinical trials in the region. For instance, in 2021, numerous technology companies in North America continued to develop and enhance their platforms and solutions for virtual clinical trials. For example, Medable, a California-based company, raised $91 million in Series C funding to further develop its decentralized trial platform, which enables remote data collection and patient engagement. Furthermore, the potential of virtual clinical trials and the digital health market, in general, has attracted significant investment in North America. Venture capital firms, pharmaceutical companies, and private investors are investing in technology providers and startups that offer solutions for virtual trials, contributing to market growth. For instance, in 2021, Science 37, a California-based company specializing in virtual trials, raised $40 million in Series D funding. The funding will support the expansion of the company’s platform and services for decentralized clinical trials.
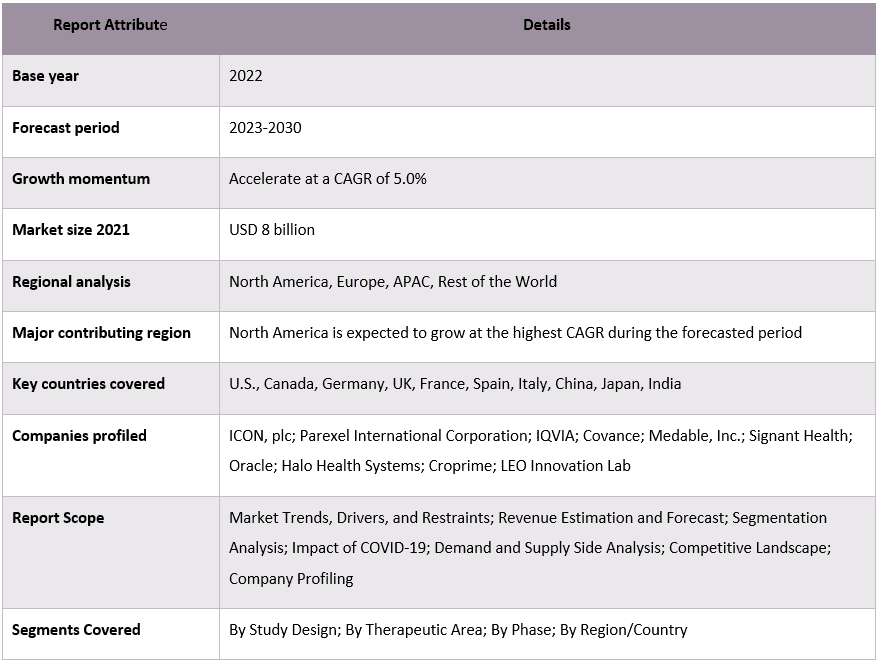
Virtual clinical trials Market Report Coverage
Reasons to buy this report:
- The study includes market sizing and forecasting analysis validated by authenticated key industry experts.
- The report presents a quick review of overall industry performance at one glance.
- The report covers an in-depth analysis of prominent industry peers with a primary focus on key business financials, product portfolios, expansion strategies, and recent developments.
- Detailed examination of drivers, restraints, key trends, and opportunities prevailing in the industry.
- The study comprehensively covers the market across different segments.
- Deep dive regional level analysis of the industry.
Customization Options:
The global Virtual clinical trials market can further be customized as per the requirement or any other market segment. Besides this, UMI understands that you may have your own business needs, hence feel free to connect with us to get a report that completely suits your requirements.
Table of Content
Research Methodology for the Virtual Clinical Trials Market Analysis (2023-2030)
Analyzing the historical market, estimating the current market, and forecasting the future market of the global Virtual clinical trials market were the three major steps undertaken to create and analyze the adoption of Virtual clinical trials in major regions globally. Exhaustive secondary research was conducted to collect the historical market numbers and estimate the current market size. Secondly, to validate these insights, numerous findings and assumptions were taken into consideration. Moreover, exhaustive primary interviews were also conducted, with industry experts across the value chain of the global Virtual clinical trials market. Post assumption and validation of market numbers through primary interviews, we employed a top-down/bottom-up approach to forecasting the complete market size. Thereafter, market breakdown and data triangulation methods were adopted to estimate and analyze the market size of segments and sub-segments of the industry pertains to. Detailed methodology is explained below:
Analysis of Historical Market Size
Step 1: In-Depth Study of Secondary Sources:
Detail secondary study was conducted to obtain the historical market size of the Virtual clinical trials market through company internal sources such as annual reports & financial statements, performance presentations, press releases, etc., and external sources including journals, news & articles, government publications, competitor publications, sector reports, third-party database, and other credible publications.
Step 2: Market Segmentation:
After obtaining the historical market size of the Virtual clinical trials market, we conducted a detailed secondary analysis to gather historical market insights and share for different segments & sub-segments for major regions. Major segments are included in the report as study design, therapeutic area, and phase. Further country-level analyses were conducted to evaluate the overall adoption of testing models in that region.
Step 3: Factor Analysis:
After acquiring the historical market size of different segments and sub-segments, we conducted a detailed factor analysis to estimate the current market size of the Virtual clinical trials market. Further, we conducted factor analysis using dependent and independent variables such as study design, therapeutic area, and phase of the virtual clinical trials market. A thorough analysis was conducted for demand and supply-side scenarios considering top partnerships, mergers and acquisitions, business expansion, and product launches in the Virtual clinical trials market sector across the globe.
Current Market Size Estimate & Forecast
Current Market Sizing: Based on actionable insights from the above 3 steps, we arrived at the current market size, key players in the global Virtual clinical trials market, and market shares of the segments. All the required percentage shares split, and market breakdowns were determined using the above-mentioned secondary approach and were verified through primary interviews.
Estimation & Forecasting: For market estimation and forecast, weights were assigned to different factors including drivers & trends, restraints, and opportunities available for the stakeholders. After analyzing these factors, relevant forecasting techniques i.e., the top-down/bottom-up approach were applied to arrive at the market forecast for 2030 for different segments and sub-segments across the major markets globally. The research methodology adopted to estimate the market size encompasses:
- The industry’s market size, in terms of revenue (USD) and the adoption rate of the Virtual clinical trials market across the major markets domestically
- All percentage shares, splits, and breakdowns of market segments and sub-segments
- Key players in the global Virtual clinical trials market in terms of products offered. Also, the growth strategies adopted by these players to compete in the fast-growing market
Market Size and Share Validation
Primary Research: In-depth interviews were conducted with the Key Opinion Leaders (KOLs) including Top Level Executives (CXO/VPs, Sales Head, Marketing Head, Operational Head, Regional Head, Country Head, etc.) across major regions. Primary research findings were then summarized, and statistical analysis was performed to prove the stated hypothesis. Inputs from primary research were consolidated with secondary findings, hence turning information into actionable insights.
Split of Primary Participants in Different Regions
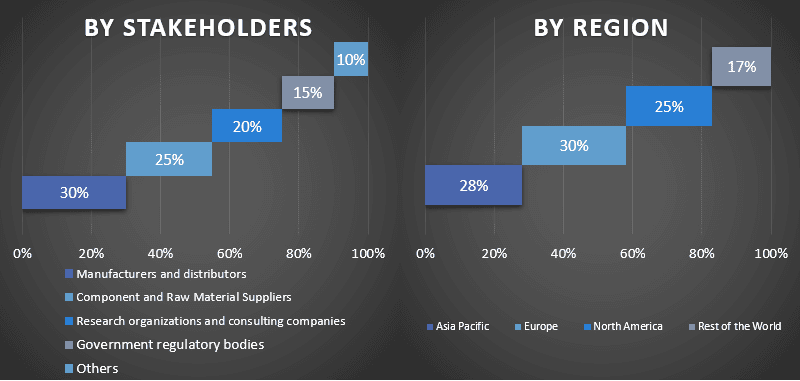
Market Engineering
The data triangulation technique was employed to complete the overall market estimation and to arrive at precise statistical numbers for each segment and sub-segment of the global virtual clinical trials market. data was split into several segments & sub-segments post studying various parameters and trends in the areas of the study design, therapeutic area, and phase in the global Virtual clinical trials market.
The main objective of the Global Virtual clinical trials Market Study
The current & future market trends of the global Virtual clinical trials market were pinpointed in the study. Investors can gain strategic insights to base their discretion for investments on the qualitative and quantitative analysis performed in the study. Current and future market trends determined the overall attractiveness of the market at a regional level, providing a platform for the industrial participant to exploit the untapped market to benefit from a first-mover advantage. Other quantitative goals of the studies include:
- Analyze the current and forecast market size of the Virtual clinical trials market in terms of value (USD). Also, analyze the current and forecast market size of different segments and sub-segments
- Segments in the study include areas of the study design, therapeutic area, and phase.
- Define and analysis of the regulatory framework for the Virtual clinical trials industry
- Analyze the value chain involved with the presence of various intermediaries, along with analyzing customer and competitor behaviors of the industry
- Analyze the current and forecast market size of the Virtual clinical trials market for the major region
- Major countries of regions studied in the report include Asia Pacific, Europe, North America, and the Rest of the World
- Company profiles of the Virtual clinical trials market and the growth strategies adopted by the market players to sustain in the fast-growing market
- Deep dive regional level analysis of the industry
Related Reports
Customers who bought this item also bought