法布里病(也称为安德森-法布里病)是一种进行性的 X 连锁遗传性糖鞘脂代谢遗传性疾病,由溶酶体 α-半乳糖苷酶 A 活性缺陷或缺失引起。它是一种毁灭性的先天性代谢缺陷,尤其是在早期阶段,细胞功能障碍和微血管病变是由溶酶体糖鞘脂沉积引起的。溶酶体外糖苷水解酶 α-半乳糖苷酶 A 的缺失或活性不足会导致球形三糖酰基神经酰胺和相关的糖鞘脂在溶酶体(普遍存在的亚细胞器)内逐渐积累。
影响儿童幸福感和表现的首发临床症状出现在儿童时期,通常在 3 岁至 10 岁之间,通常女孩比男孩晚几年。随着年龄的增长,重要器官系统会逐渐受到损害,导致男女器官衰竭。终末期肾病和危及生命的心血管或脑血管并发症会限制预期寿命。临床症状是多系统的、异质的和进行性的。
治疗产品的划分
法布里病的生化诊断是通过测量从外周血、培养的成纤维细胞或从滤纸血斑中提取的样本中 α-gal A 的活性来确定的。FD 的诊断可以通过仔细的临床和仪器检查,以及家族史数据和对遗传和酶学分析的准确解释得出。通过分子遗传学测试鉴定半合子 GLA 致病变异体可以确诊男性先证者。
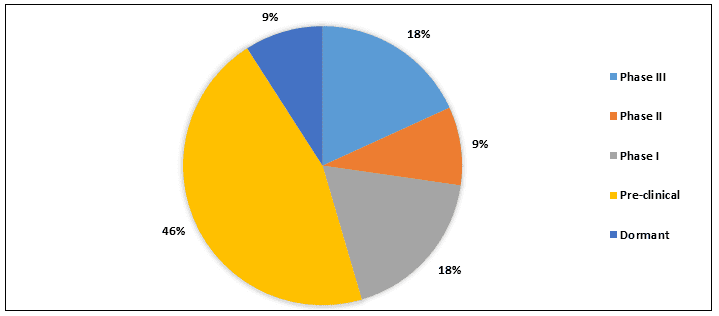
法布里病的治疗管线包括大约 9+ 种处于不同开发阶段的产品。目前,2+ 种药物处于 III 期开发阶段,主要药物处于临床前阶段。
分析的顶级公司
一些主要参与者包括 Amicus Therapeutics;Evotec;Freeline;Greenovation Biotech;Idorsia Pharmaceuticals;Moderna;Pharming;Protalix Biotherapeutics;Resverlogix Corp;Sangamo Therapeutics 和 Sanofi Genzyme。
研究范围:
提供法布里病在完整产品开发周期(包括所有临床和非临床阶段)的治疗管线活动概览
该报告包括法布里病治疗产品的详细概况,重点介绍了开发活动,包括许可和合作协议、已颁发专利、指定、技术和化学信息
按阶段、产品类型、分子类型和给药途径对活性管线产品进行治疗评估
报告中包含休眠产品的详细概况
购买理由:
法布里病管线提供了药物的详细概况。报告中提供的分析是深入的二级研究和行业关键意见领袖投入的结合
该报告快速回顾了当前关于该适应症药物开发的情况
该报告深入分析了杰出的行业同行,主要关注公司整合、指定、技术、协议和关于该疗法的专利
详细检查行业中普遍存在的诊断、治疗和指南
借助推出时间表检查行业吸引力
该研究全面涵盖了处于不同开发阶段的药物市场
广泛的治疗领域领域知识通过确定非活性或停产产品背后的原因来支持客户在关于其治疗组合的决策过程
定制选项:
法布里病管线分析报告可以定制到国家层面或任何其他竞争细分市场。除此之外,UMI 理解您可能有自己的业务需求,因此我们也为客户提供完全定制的解决方案。
目录
对法布里病治疗药物的管线开发活动概述分析不仅限于药物描述和开发活动,还侧重于临床和非临床结果。它还包括指定、公司合并&许可交易、赠款、技术和专利详细信息。法布里病产品的治疗细分是根据药物的开发阶段进行的。该报告包括比较性的管线疗法评估,以及按开发阶段、疗法类型、分子类型和给药途径对管线产品进行的详细药物概况。该报告还包括对即将推出的管线疗法的预测发布时间表。此外,分析师的见解也被重点关注,以提供有关当前市场情况的摘要。完整的报告中阐明了休眠产品的详细资料,以及它们休眠的相关原因。
二级研究
进行了详细的二级研究,通过公司内部来源(如年度报告、业绩演示文稿、新闻稿等)以及外部来源(包括行业期刊、新闻&文章、政府出版物、竞争对手出版物、行业报告、监管机构出版物、安全标准组织、第三方数据库和其他可信出版物)获得法布里病治疗药物的管线分析。
正在对内部和外部来源进行二级研究,以获取与每个市场相关的定性和定量信息。
- 公司网站、年度报告、季度报告、财务报告、经纪人报告、投资者演示文稿和美国证券交易委员会 (SEC) 文件
- 行业贸易期刊和其他文献
- 国家政府文件、统计数据库和市场报告
- 特定于在市场中运营的公司的新闻文章、新闻稿和网络广播
- 多个专利和临床试验数据库
法布里病 – 2019 年管线分析的主要目标
该研究指出了法布里病目前的管线趋势。投资者可以获得战略见解,以便根据研究中进行的定性和定量分析来做出投资判断。当前和未来的管线趋势将决定市场的整体吸引力,为行业参与者提供一个平台,以利用尚未开发的市场,从而获得先发优势。该报告的定量目标包括:
- 概述法布里病在整个产品开发周期(包括所有临床和非临床阶段)的治疗管线活动
- 分析法布里病治疗产品的详细资料,重点介绍开发活动,包括许可&合作协议、已颁发的专利、指定、技术和化学性质信息
- 按阶段、产品类型、分子类型和给药途径对活性管线产品进行治疗评估
相关 报告
购买此商品的客户也购买了