- Home
- Chi siamo
- Settore
- Servizi
- Lettura
- Contattaci
Mercato della terapia genica: analisi e previsioni (2025-2033)
Focus su vettore {Vettore virale (Retrovirale e Adeno-associato) e Vettore non virale (Oligonucleotidi)}; Tipo di gene (Antigene, Citochina, Recettore e Altri); Indicazione (Oncologia, Malattie genetiche rare, Cardiovascolare, Neurologia e Altri); Metodo di somministrazione (In-Vivo e Ex-Vivo); e Regione/Paese.
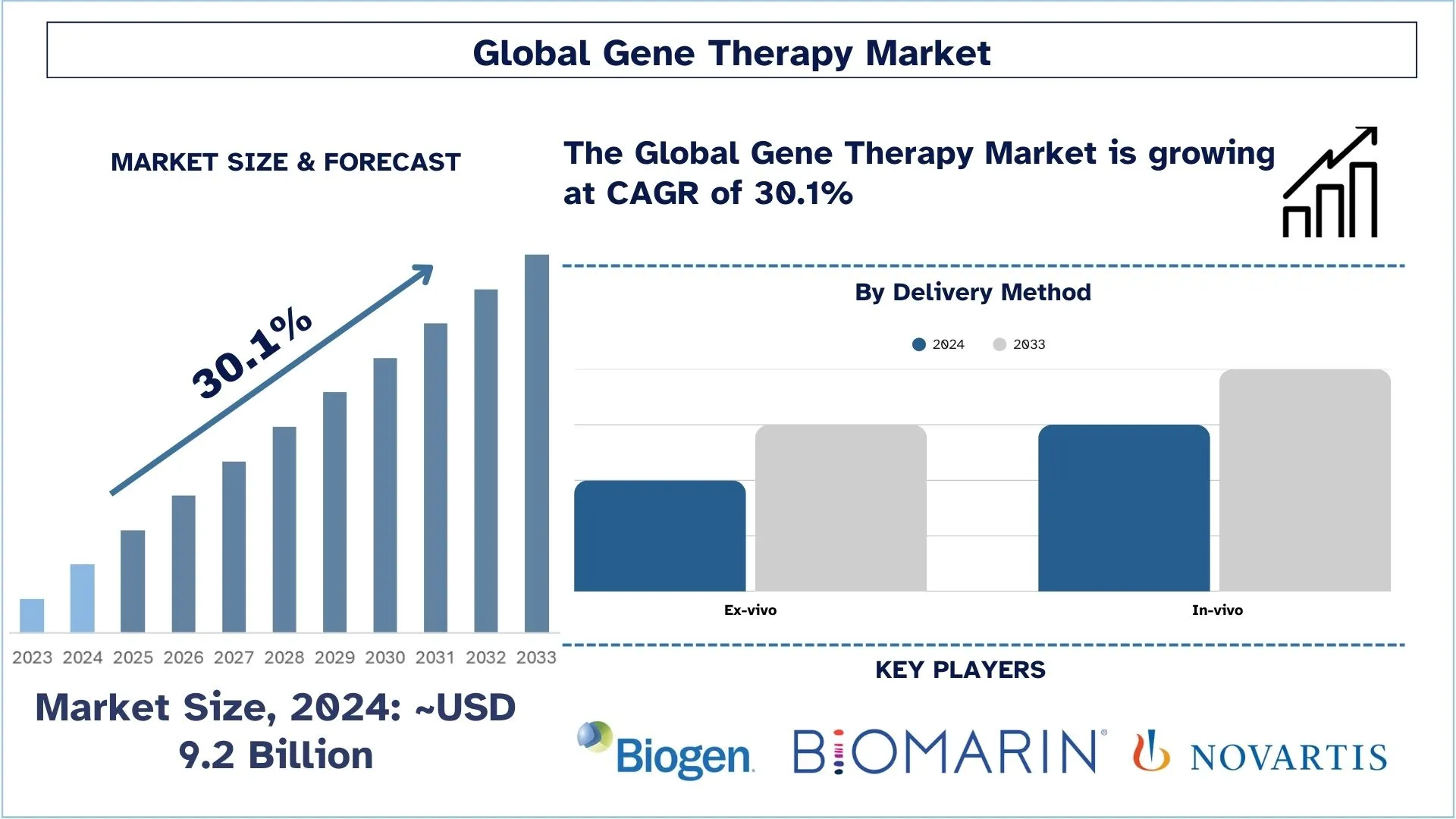
Dimensioni e previsioni del mercato della terapia genica
Il mercato della terapia genica è stato valutato a circa 9,2 miliardi di dollari USA nel 2024 e si prevede che crescerà a un CAGR sostanziale di circa il 30,1% durante il periodo di previsione (2025-2033), grazie alla crescente pipeline di terapia genica.
Analisi del mercato della terapia genica
Il mercato della terapia genica è in costante crescita e i suoi tassi di crescita aumentano costantemente a causa dei costanti sviluppi nella scienza genetica e nella biotecnologia che influenzano il trattamento delle malattie. C'è un'idea condivisa di terapia genica in cui i geni difettosi vengono riparati o sostituiti per offrire una cura a lungo termine per le malattie. Questo viene applicato in oncologia, disturbi genetici rari, malattie neurologiche e oculari, tra le altre, a causa della sua efficacia.
Il mercato è trainato principalmente da fattori quali i preparativi delle industrie biofarmaceutiche, l'approvazione dei trattamenti genoregolati e il patrocinio delle autorità statali. I progressi nelle tecnologie vettoriali, nelle tecniche di produzione e nei sistemi di somministrazione sono in costante crescita e stanno anche migliorando gli usi clinici dei vettori. Partnership, operazioni e studi di ricerca precisi stanno costantemente guidando lo sviluppo della terapia genica come uno dei campi più significativi nel mondo contemporaneo.
Tendenze del mercato della terapia genica
Questa sezione discute le principali tendenze del mercato che influenzano i vari segmenti del mercato della terapia genica, come identificato dai nostri esperti di ricerca.
Espansione delle applicazioni oltre le malattie rare
Una tendenza significativa nella struttura del mercato della terapia genica a livello globale è il passaggio dal trattamento di malattie rare o congenite all'intervento in malattie più comuni e multifattoriali. La prima applicazione della terapia genica è stata quella di trattare alcuni disturbi monogenici poiché i loro obiettivi sono chiari marcatori genetici e le esigenze mediche non erano soddisfatte; tuttavia, ora viene utilizzata in cancro, malattie cardiovascolari e neurologiche. Pertanto, ci sono stati progressi nelle tecnologie vettoriali, migliori strumenti di editing genetico e un tasso di successo dei trial nella ricerca clinica. Prevedevano che questa tendenza avrebbe contribuito all'ampia crescita della popolazione di pazienti e alle prospettive di mercato delle terapie geniche in tutto il mondo man mano che la scienza si sviluppa.
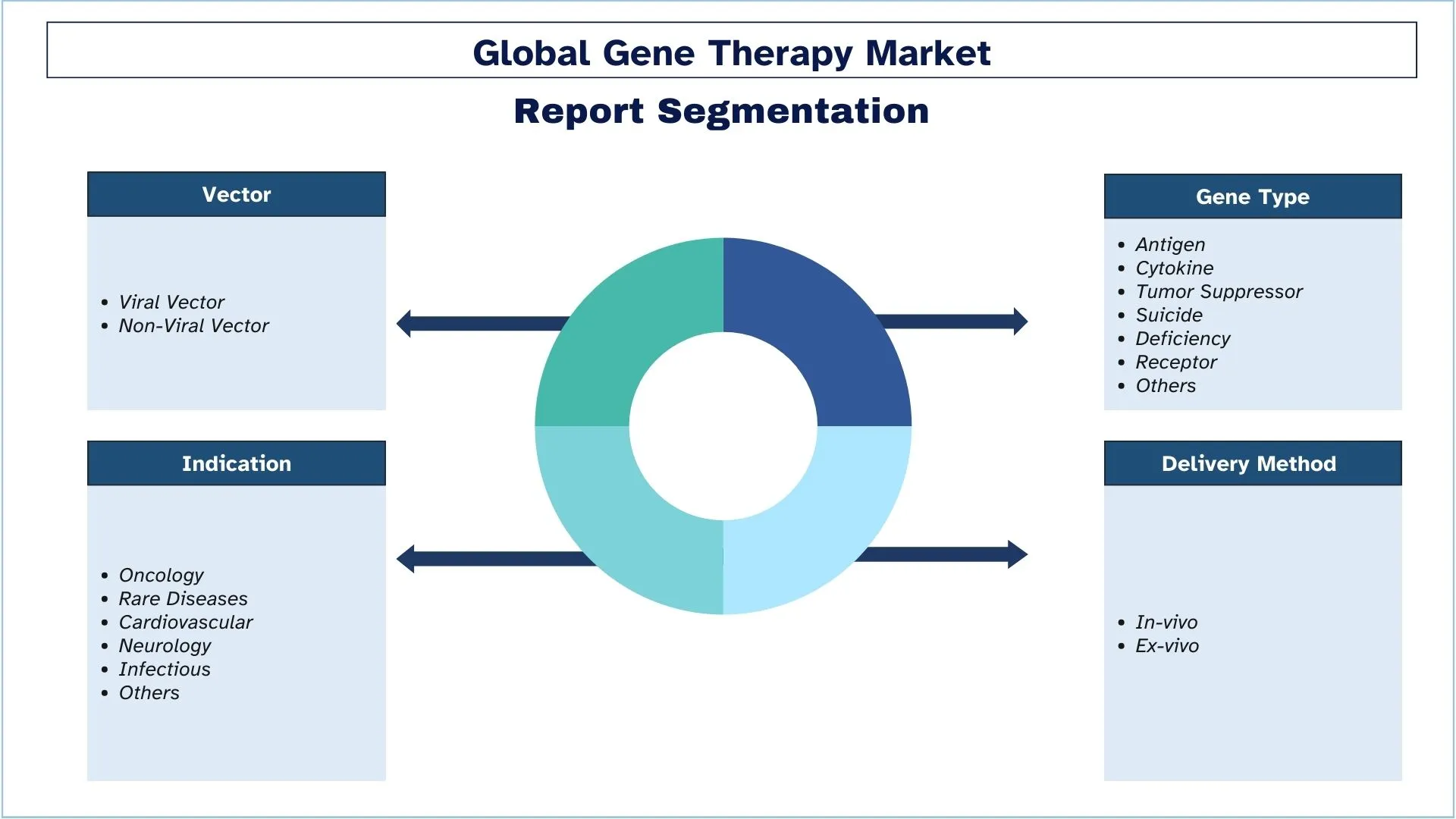
Segmentazione del settore della terapia genica
Questa sezione fornisce un'analisi delle principali tendenze in ogni segmento del rapporto globale sulla terapia genica, insieme alle previsioni a livello globale, regionale e nazionale per il 2025-2033.
Il segmento dei vettori virali detiene la quota maggiore del mercato della terapia genica.
In base al vettore, il mercato è segmentato in vettore virale (retrovirale e adeno-associato) e vettore non virale (oligonucleotidi). Il vettore virale ha detenuto una quota dominante del mercato nel 2022. I vettori lentivirali, derivati dal virus dell'immunodeficienza umana, sono stati ampiamente studiati e ottimizzati negli ultimi due decenni. I vettori lentivirali di terza generazione, auto-inattivanti, sono stati recentemente utilizzati in molteplici sperimentazioni cliniche per introdurre geni nelle cellule staminali ematopoietiche per correggere le immunodeficienze primarie e le emoglobinopatie. Le terapie con cellule CAR T ingegnerizzate utilizzando vettori lentivirali hanno dimostrato un notevole successo clinico in pazienti con neoplasie a cellule B, portando all'approvazione normativa della prima terapia cellulare geneticamente modificata utilizzando vettori lentivirali.
Il segmento ex vivo dovrebbe registrare un CAGR più elevato rispetto al mercato della terapia genica.
Per metodo di somministrazione, il mercato è suddiviso in in vivo ed ex vivo. Si prevede che il segmento ex vivo crescerà con un CAGR elevato durante il periodo di previsione. L'espressione del gene e la salute delle cellule trasfettate possono essere verificate prima che venga introdotta al paziente tramite la somministrazione ex vivo. Questo metodo può essere utile per le terapie in cui i composti devono essere rilasciati o espressi da specifici tipi di cellule che possono essere selezionati sulla piastra di coltura prima del trapianto. In ex vivo, le cellule vengono modificate al di fuori del corpo del paziente e la versione corretta viene ritrapiantata nel paziente. Le cellule vengono trattate con un vettore di terapia genica virale o non virale che trasporta la copia corretta del gene.
Il Nord America detiene una quota significativa del mercato nel 2024.
Il mercato nordamericano della terapia genica detiene la posizione di mercato più progressiva a livello globale in termini di innovazione e crescita, grazie alla maggiore attenzione alla ricerca e sviluppo, alla legislazione di supporto e a un elevato livello di investimenti in biotecnologie. Gode quindi di un ambiente favorevole in cui agenzie come la FDA approvano nuove terapie geniche per una commercializzazione più rapida. La regione ha un numero enorme di importanti aziende farmaceutiche e biotecnologiche eccezionali, oltre a una crescente attenzione al trattamento di malattie come disturbi genetici, cancro e altre malattie rare. Alcuni di questi includono alti costi di trattamento e un ambiente normativo complesso, ma la crescita è costante grazie all'integrazione della terapia genica in applicazioni cliniche e commerciali.
Gli Stati Uniti dominano il mercato nordamericano della terapia genica
La quota di mercato della terapia genica è in crescita negli Stati Uniti grazie all'aumento delle spese per attività di ricerca e sviluppo, a una regolamentazione favorevole e a un recente aumento delle approvazioni FDA di nuove terapie per condizioni genetiche e altre condizioni rare o croniche. La terapia genica sta emergendo come un nuovo paradigma per il trattamento in quanto trova i suoi usi in oncologia, neurologia e disturbi genetici, e molti altri. Sebbene il mercato sembri promettente, problemi come gli elevati costi di sviluppo, i complessi processi di produzione e l'accesso al trattamento possono essere considerati i principali fattori che influenzano lo sviluppo del mercato in futuro.
Panorama competitivo del settore della terapia genica
Il mercato della terapia genica è competitivo, con diversi attori globali e internazionali. I principali attori stanno adottando diverse strategie di crescita per migliorare la loro presenza sul mercato, come partnership, accordi, collaborazioni, lanci di nuovi prodotti, espansioni geografiche e fusioni e acquisizioni.
Le principali aziende di terapia genica
Alcuni dei principali attori che operano nel mercato sono Bluebird Bio (Carlyle e SK Capital), Biogen, BioMarin Pharmaceuticals, Celgene Corporation (Bristol Myers Squibb), Gilead Sciences, Novartis, Orchard Therapeutics (Kyowa Kirin company), Regenxbio, Spark Therapeutics (Roche) e uniQure.
Sviluppi recenti nel mercato della terapia genica
Nel gennaio 2024, Biogen e Ginkgo Bioworks hanno annunciato il completamento della loro collaborazione sulla terapia genica che coinvolge vettori a base di AAV. Si prevede che ciò alimenterà la domanda di terapie geniche nei prossimi anni.
Nel dicembre 2023, l'Agenzia svizzera per i prodotti terapeutici ha concesso l'approvazione a Libmeldy per il trattamento della leucodistrofia metacromatica ad esordio precoce.
Nel maggio 2023, Krystal Biotech è stata approvata per la terapia genica VYJUVEK per il trattamento della epidermolisi bollosa distrofica
Copertura del rapporto sul mercato della terapia genica
Dettagli | |
Anno base | 2024 |
Periodo di previsione | 2025-2033 |
Slancio di crescita | Accelerare a un CAGR del 30,1% |
Dimensione del mercato 2024 | 9,2 miliardi di dollari USA |
Analisi regionale | Nord America, Europa, Asia-Pacifico, Resto del mondo |
Regione che contribuisce in modo significativo | Si prevede che il Nord America crescerà al CAGR più elevato durante il periodo previsto. |
Paesi chiave coperti | Stati Uniti, Canada, Germania, Francia, Regno Unito, Spagna, Italia, Cina, Giappone e India |
bluebird bio (Carlyle e SK Capital), Biogen, BioMarin Pharmaceuticals, Celgene Corporation (Bristol Myers Squibb), Gilead Sciences, Novartis, Orchard Therapeutics (Kyowa Kirin company), Regenxbio, Spark Therapeutics (Roche) e uniQure | |
Ambito del rapporto | Tendenze di mercato, fattori trainanti e frenanti; Stima e previsione dei ricavi; Analisi di segmentazione; Analisi della domanda e dell'offerta; Panorama competitivo; Profilazione aziendale |
Segmenti coperti | Per vettore, per tipo di gene, per indicazione, per metodo di somministrazione, per regione/paese |
Motivi per acquistare il rapporto di mercato sulla terapia genica:
Lo studio include la stima delle dimensioni del mercato e l'analisi delle previsioni convalidata da esperti chiave del settore autenticati.
Il rapporto presenta una rapida panoramica delle prestazioni complessive del settore.
Il rapporto copre un'analisi approfondita dei principali attori del settore con particolare attenzione ai dati finanziari aziendali chiave, ai portafogli di prodotti, alle strategie di espansione e agli sviluppi recenti.
Esame dettagliato dei fattori trainanti, dei vincoli, delle tendenze chiave e delle opportunità prevalenti nel settore.
Lo studio copre in modo completo il mercato in diversi segmenti.
Analisi approfondita a livello regionale del settore.
Opzioni di personalizzazione:
Il mercato globale della terapia genica può essere ulteriormente personalizzato in base alle esigenze o a qualsiasi altro segmento di mercato. Inoltre, UnivDatos comprende che potresti avere le tue esigenze aziendali; quindi, non esitare a contattarci per ottenere un rapporto che soddisfi completamente le tue esigenze.
Indice
Metodologia di ricerca per l'analisi del mercato della terapia genica (2023-2033)
Abbiamo analizzato il mercato storico, stimato il mercato attuale e previsto il mercato futuro del mercato globale della terapia genica per valutarne l'applicazione nelle principali regioni del mondo. Abbiamo condotto un'esauriente ricerca secondaria per raccogliere dati storici sul mercato e stimare le dimensioni attuali del mercato. Per convalidare queste intuizioni, abbiamo attentamente esaminato numerosi risultati e ipotesi. Inoltre, abbiamo condotto interviste primarie approfondite con esperti del settore in tutta la catena del valore della terapia genica. Dopo aver convalidato i dati di mercato attraverso queste interviste, abbiamo utilizzato approcci top-down e bottom-up per prevedere le dimensioni complessive del mercato. Abbiamo quindi impiegato metodi di scomposizione del mercato e di triangolazione dei dati per stimare e analizzare le dimensioni del mercato dei segmenti e dei sottosegmenti del settore.
Ingegneria del mercato
Abbiamo impiegato tecniche di triangolazione dei dati per finalizzare la stima complessiva del mercato e ricavare numeri statistici precisi per ogni segmento e sottosegmento del mercato globale della terapia genica. Abbiamo suddiviso i dati in diversi segmenti e sottosegmenti analizzando vari parametri e tendenze, tra cui vettore, tipo di gene, indicazione, metodo di somministrazione e regioni all'interno del mercato globale della terapia genica.
L'obiettivo principale dello studio sul mercato globale della terapia genica è
Lo studio identifica le tendenze attuali e future nel mercato globale della terapia genica, fornendo approfondimenti strategici per gli investitori. Mette in evidenza l'attrattiva del mercato regionale, consentendo ai partecipanti del settore di attingere a mercati inesplorati e ottenere un vantaggio da pioniere. Altri obiettivi quantitativi degli studi includono:
Analisi delle dimensioni del mercato:Valutare le dimensioni attuali e previste del mercato globale della terapia genica e dei suoi segmenti in termini di valore (USD).
Segmentazione del mercato della terapia genica:Lo studio segmenta il mercato per vettore, tipo di gene, indicazione, metodo di somministrazione e regione.
Quadro normativo e analisi della catena del valore:Esaminare il quadro normativo, la catena del valore, il comportamento dei clienti e il panorama competitivo del settore della terapia genica.
Analisi regionale:Condurre un'analisi regionale dettagliata per aree chiave come Asia Pacifico, Europa, Nord America e Resto del mondo.
Profili aziendali e strategie di crescita:Profili aziendali del mercato della terapia genica e le strategie di crescita adottate dai leader di mercato per sostenere il mercato in rapida crescita.
Domande frequenti FAQ
Q1: Qual è l'attuale dimensione e il potenziale di crescita del mercato della terapia genica?
Il mercato globale della terapia genica è valutato a circa 9,2 miliardi di dollari USA nel 2024 e si prevede che crescerà a un robusto CAGR del 30,1% fino al 2033, trainato dai progressi tecnologici e dall'espansione delle applicazioni cliniche.
Q2: Quali sono i fattori trainanti per la crescita del mercato della terapia genica?
La crescente prevalenza di disturbi genetici e le esigenze cliniche insoddisfatte stanno guidando gli investimenti e l'innovazione nella terapia genica come potenziale opzione di trattamento a lungo termine o curativo.
Q3: Quale mercato detiene la quota maggiore del mercato della terapia genica per indicazione?
La categoria Oncologia detiene attualmente la quota di mercato maggiore nel segmento di indicazione.
Q4: Quali sono le principali tendenze nel mercato della terapia genica?
C'è uno spostamento crescente verso le terapie geniche in vivo e i sistemi di somministrazione avanzati, che migliorano la precisione e riducono i rischi associati agli approcci precedenti.
Q5: Quale regione dominerà il mercato della terapia genica?
La regione del Nord America domina attualmente il mercato globale della terapia genica.
Q6: Quali sono le maggiori sfide nel mercato della terapia genica?
Gli elevati costi di sviluppo e produzione, insieme a percorsi normativi complessi, continuano a limitare la scalabilità e l'accessibilità delle terapie geniche.
Q7: Chi sono i principali attori nel mercato globale della terapia genica?
Le aziende leader che guidano l'innovazione nella terapia genica includono:
• bluebird bio (Carlyle and SK Capital)
• Biogen
• BioMarin Pharmaceuticals
• Celgene Corporation (Bristol Myers Squibb)
• Gilead Sciences
• Novartis
• Orchard Therapeutics (Kyowa Kirin company)
• Regenxbio
• Spark Therapeutics (Roche)
• uniQure
Q8: Quali sono le principali considerazioni normative per lo sviluppo e la commercializzazione della terapia genica?
Agenzie normative come la FDA e l'EMA hanno stabilito percorsi specifici per le terapie geniche, concentrandosi sulla sicurezza, sull'efficacia a lungo termine e sulla qualità della produzione. Le aziende e gli investitori devono affrontare processi di approvazione complessi, requisiti di sorveglianza post-marketing e linee guida in evoluzione per garantire la conformità e il successo sul mercato.
Q9: In che modo le partnership e le attività di M&A stanno influenzando il mercato della terapia genica?
Collaborazioni strategiche, accordi di licenza e fusioni e acquisizioni stanno accelerando l'innovazione e l'espansione dell'accesso al mercato nello spazio della terapia genica. Le aziende farmaceutiche più grandi stanno collaborando sempre più con le aziende biotech per rafforzare le pipeline, condividere i costi di ricerca e sviluppo e accelerare la commercializzazione di terapie avanzate.
Correlati Report
I clienti che hanno acquistato questo articolo hanno acquistato anche










