Рынок устройств респираторной эндотерапии: текущий анализ и прогноз (2023-2030 гг.)
Акцент на устройствах [Диагностические устройства (трансбронхиальная аспирация, биопсийные щипцы, цитологические щетки и др.) и Терапевтические устройства (такие как бронхоскопы, системы бронхиальной термопластики, баллонные катетеры для дилатации легких, стенты и др.)]; Конечный потребитель (Больницы, Диагностические центры и Амбулаторные хирургические центры); и Регион/Страна
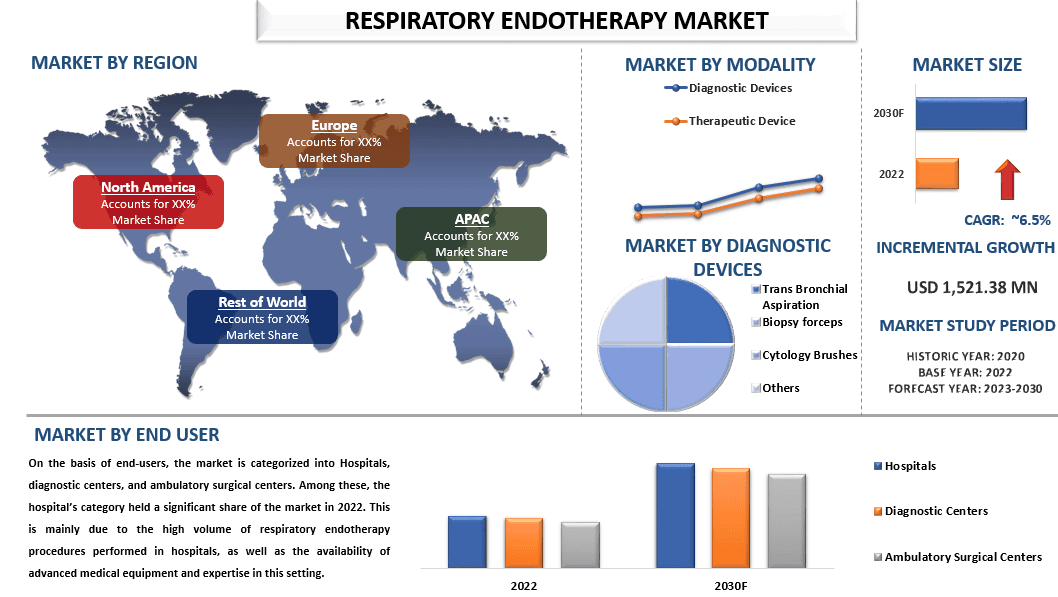
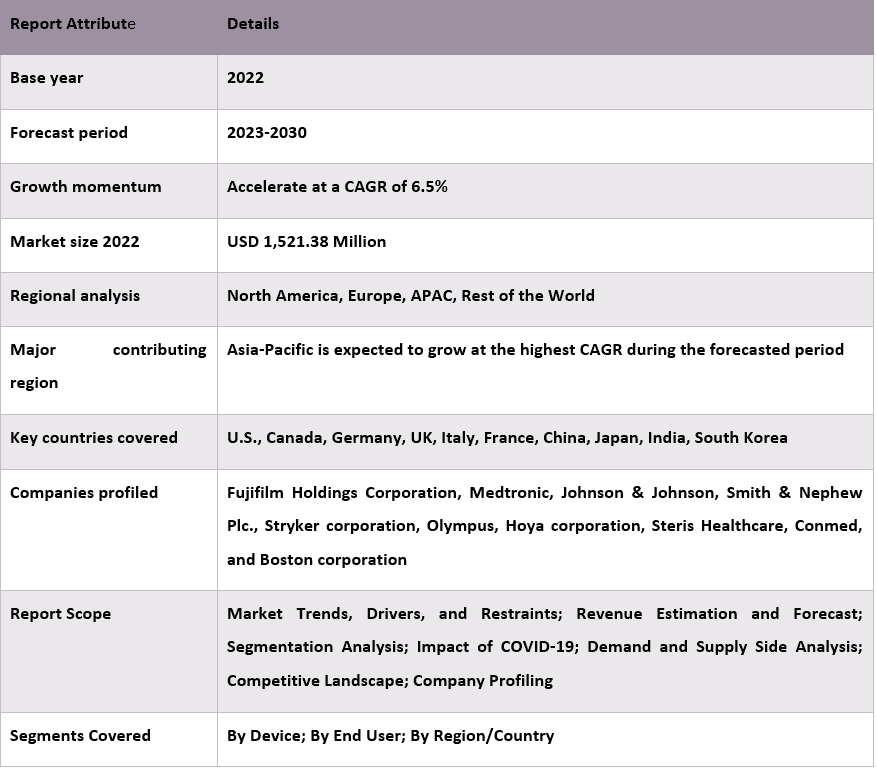
Объем рынка устройств для эндотерапии органов дыхания в 2022 году оценивался в 1 521,38 млн долларов США, и ожидается, что в период с 2023 по 2030 год он будет расти со среднегодовым темпом роста около 6,5%. Эндотерапия органов дыхания включает в себя оценку трахеи, бронхов и части легких и в основном используется для сбора образцов для клинических и анатомических патологоанатомических лабораторных исследований. Это очень эффективная процедура в диагностике опухолей и инфекционных заболеваний. Поскольку распространенность респираторных заболеваний, включая астму, ХОБЛ, бронхит, рак легких и т. д., постоянно растет, это приводит к значительному росту спроса на респираторные устройства, включая устройства для эндотерапии органов дыхания. Движущими силами рынка устройств для эндотерапии органов дыхания являются, главным образом, увеличение количества процедур эндотерапии, растущая распространенность респираторных заболеваний, технологический прогресс в оборудовании для эндотерапии и повышение осведомленности о преимуществах ранней диагностики. Например, по данным Управления по санитарному надзору за качеством пищевых продуктов и медикаментов США (FDA), в США ежегодно проводится более 500 000 бронхоскопий.
Некоторые из основных игроков, работающих на рынке, — Fujifilm Holdings Corporation, Medtronic, Johnson & Johnson, Smith & Nephew Plc., Stryker corporation, Olympus, Hoya corporation, Steris Healthcare, Conmed и Boston corporation и другие.
Информация, представленная в отчете
«Ожидается, что среди устройств терапевтические устройства будут доминировать в течение прогнозируемого периода».
В зависимости от устройства рынок сегментирован на диагностические и терапевтические устройства. В 2022 году на рынке доминировала категория терапевтических устройств, на долю которой приходилось 60,15% от общего объема рынка. Эндотерапия органов дыхания рекомендуется, когда необходимо аспирировать толстые участки, вызывающие ателектаз легких, для удаления инородных тел из легких и уменьшения или удаления опухолей в трахее или бронхах. Рост числа случаев заболеваний легких и запуск технологически продвинутых бронхоскопов стимулируют рынок сегмента терапевтических устройств.
«Среди конечных пользователей доминирующую долю в 2022 году занимал сегмент больниц».
На основе конечных пользователей рынок подразделяется на больницы, диагностические центры и амбулаторные хирургические центры. Среди них значительную долю рынка в 2022 году занимала категория больниц. Это в основном связано с большим объемом процедур эндотерапии органов дыхания, выполняемых в больницах, а также с наличием современного медицинского оборудования и опыта в этой области. Кроме того, растущий спрос на минимально инвазивные процедуры и растущая доступность современного медицинского оборудования и технологий также способствуют росту рынка.
«Ожидается, что в Азиатско-Тихоокеанском регионе рынок устройств для эндотерапии органов дыхания будет расти с самым высоким среднегодовым темпом роста в течение прогнозируемого периода».
Ожидается, что в Азиатско-Тихоокеанском регионе рынок устройств для эндотерапии органов дыхания будет расти с самым высоким среднегодовым темпом роста в течение прогнозируемого периода. Регион состоит из быстро развивающихся стран, таких как Китай, Япония и Индия, из-за чего растет спрос на передовые диагностические и терапевтические технологии. Кроме того, в Азиатско-Тихоокеанском регионе проживает более 60% населения мира, и этот фактор также делает регион наиболее привлекательным рынком для роста индустрии устройств для эндотерапии органов дыхания. Наряду с этим, рост числа случаев респираторных заболеваний, таких как рак легких, астма, ХОБЛ, бронхит и т. д., в Азии также стимулирует рост рынка. Например, по данным ВОЗ, рак легких является ведущим типом рака среди населения Азии, и около 1,3 миллиона новых случаев рака легких было зарегистрировано в 2020 году.
Охват отчета о рынке устройств для эндотерапии органов дыхания
Причины купить этот отчет:
- Исследование включает в себя анализ размеров рынка и прогнозирование, подтвержденные проверенными ключевыми экспертами отрасли.
- Отчет представляет собой краткий обзор общей производительности отрасли с первого взгляда.
- Отчет охватывает углубленный анализ видных представителей отрасли с уделением особого внимания основным финансовым показателям бизнеса, портфелям продуктов, стратегиям расширения и последним событиям.
- Подробное изучение движущих сил, ограничений, ключевых тенденций и возможностей, преобладающих в отрасли.
- Исследование всесторонне охватывает рынок по различным сегментам.
- Глубокий анализ отрасли на региональном уровне.
Варианты настройки:
Глобальный рынок устройств для эндотерапии органов дыхания может быть дополнительно настроен в соответствии с требованиями или любым другим сегментом рынка. Кроме того, UMI понимает, что у вас могут быть свои собственные потребности в бизнесе, поэтому не стесняйтесь обращаться к нам, чтобы получить отчет, который полностью соответствует вашим требованиям.
Содержание
Методология исследования для анализа рынка респираторных эндотерапевтических устройств (2023-2030 гг.)
Анализ исторического рынка, оценка текущего рынка и прогнозирование будущего рынка глобального рынка респираторных эндотерапевтических устройств были тремя основными этапами, предпринятыми для создания и анализа внедрения респираторных эндотерапевтических устройств в основных регионах мира. Было проведено исчерпывающее вторичное исследование для сбора исторических данных о рынке и оценки текущего размера рынка. Во-вторых, для подтверждения этих выводов было принято во внимание множество результатов и предположений. Кроме того, были проведены исчерпывающие первичные интервью с отраслевыми экспертами по всей цепочке создания стоимости глобального рынка респираторных эндотерапевтических устройств. После предположения и подтверждения рыночных показателей посредством первичных интервью мы использовали подход "сверху вниз/снизу вверх" для прогнозирования общего размера рынка. После этого были приняты методы декомпозиции рынка и триангуляции данных для оценки и анализа размера рынка сегментов и подсегментов соответствующей отрасли. Подробная методология описана ниже:
Анализ исторических размеров рынка
Этап 1: Углубленное изучение вторичных источников:
Было проведено подробное вторичное исследование для получения исторических данных о размере рынка респираторных эндотерапевтических устройств из внутренних источников компании, таких как годовые отчеты и финансовые отчетности, презентации о деятельности, пресс-релизы и т. д., и внешних источников, включая журналы, новости и статьи, правительственные публикации, публикации конкурентов, отраслевые отчеты, сторонние базы данных и другие достоверные публикации.
Этап 2: Сегментация рынка:
После получения исторических данных о размере рынка респираторных эндотерапевтических устройств мы провели подробный вторичный анализ для сбора исторических рыночных данных и доли для различных сегментов и подсегментов для основных регионов. Основные сегменты, включенные в отчет, - это модальность, тип услуг и специалист. Был проведен дальнейший анализ на уровне стран для оценки общего внедрения моделей тестирования в этом регионе.
Этап 3: Факторный анализ:
После получения исторических данных о размере рынка различных сегментов и подсегментов мы провели подробный факторный анализ для оценки текущего размера рынка респираторных эндотерапевтических устройств. Кроме того, мы провели факторный анализ с использованием зависимых и независимых переменных, таких как модальность, тип услуг и специалист рынка респираторных эндотерапевтических устройств. Был проведен тщательный анализ сценариев спроса и предложения с учетом ведущих партнерств, слияний и поглощений, расширения бизнеса и запуска продуктов в секторе рынка респираторных эндотерапевтических устройств по всему миру.
Оценка и прогноз текущего размера рынка
Определение текущего размера рынка: На основе практически применимых результатов, полученных на основе вышеуказанных 3 этапов, мы определили текущий размер рынка, ключевых игроков на глобальном рынке респираторных эндотерапевтических устройств и доли рынка сегментов. Все необходимые процентные доли, разбивки и декомпозиции рынка были определены с использованием вышеупомянутого вторичного подхода и были проверены посредством первичных интервью.
Оценка и прогнозирование: Для оценки и прогнозирования рынка различным факторам, включая драйверы и тенденции, ограничения и возможности, доступные для заинтересованных сторон, были присвоены веса. После анализа этих факторов были применены соответствующие методы прогнозирования, т. е. подход "сверху вниз/снизу вверх", чтобы получить прогноз рынка на 2030 год для различных сегментов и подсегментов на основных рынках мира. Методология исследования, принятая для оценки размера рынка, включает в себя:
- Размер рынка отрасли с точки зрения выручки (в долларах США) и уровень внедрения респираторных эндотерапевтических устройств на основных рынках внутри страны
- Все процентные доли, разбивки и декомпозиции рыночных сегментов и подсегментов
- Ключевые игроки на глобальном рынке респираторных эндотерапевтических устройств с точки зрения предлагаемых продуктов. Кроме того, стратегии роста, принятые этими игроками для конкуренции на быстрорастущем рынке
Подтверждение размера и доли рынка
Первичное исследование: Были проведены углубленные интервью с ключевыми лидерами мнений (KOL), включая руководителей высшего звена (CXO/VP, руководители отдела продаж, руководители отдела маркетинга, руководители операционного отдела, региональные руководители, руководители страны и т. д.) в основных регионах. Затем результаты первичного исследования были обобщены, и был проведен статистический анализ для доказательства заявленной гипотезы. Данные, полученные в результате первичного исследования, были объединены с вторичными результатами, превратив, таким образом, информацию в практически применимые выводы.
Разбивка первичных участников по различным регионам

Инжиниринг рынка
Метод триангуляции данных был использован для завершения общей оценки рынка и получения точных статистических данных для каждого сегмента и подсегмента глобального рынка респираторных эндотерапевтических устройств. Данные были разделены на несколько сегментов и подсегментов после изучения различных параметров и тенденций в областях модальности, типа услуг и специалиста на глобальном рынке респираторных эндотерапевтических устройств.
Основная цель исследования глобального рынка респираторных эндотерапевтических устройств
В исследовании были точно определены текущие и будущие рыночные тенденции глобального рынка респираторных эндотерапевтических устройств. Инвесторы могут получить стратегические выводы, чтобы основывать свое усмотрение для инвестиций на качественном и количественном анализе, выполненном в исследовании. Текущие и будущие рыночные тенденции определили общую привлекательность рынка на региональном уровне, предоставив платформу для промышленного участника для использования неиспользованного рынка, чтобы извлечь выгоду из преимущества первопроходца. Другие количественные цели исследований включают в себя:
- Анализ текущего и прогнозируемого размера рынка респираторных эндотерапевтических устройств в стоимостном выражении (доллары США). Кроме того, анализ текущего и прогнозируемого размера рынка различных сегментов и подсегментов
- Сегменты в исследовании включают области модальности, типа услуг и специалиста.
- Определение и анализ нормативно-правовой базы для отрасли респираторных эндотерапевтических устройств
- Анализ цепочки создания стоимости, связанной с наличием различных посредников, наряду с анализом поведения клиентов и конкурентов отрасли
- Анализ текущего и прогнозируемого размера рынка респираторных эндотерапевтических устройств для основного региона
- Основные страны регионов, изученные в отчете, включают Азиатско-Тихоокеанский регион, Европу, Северную Америку и остальной мир
- Профили компаний рынка респираторных эндотерапевтических устройств и стратегии роста, принятые участниками рынка для поддержания устойчивости на быстрорастущем рынке
- Углубленный анализ отрасли на региональном уровне
Связанные Отчеты
Клиенты, купившие этот товар, также купили