- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
Pharmacovigilance Software Market: Current Analysis and Forecast (2022-2028)
Emphasis on Functionality (Adverse Event Reporting Software, Drug Safety Audits Software, Issue Tracking Software, and Fully Integrated Software); Mode of Delivery (On-Premises Delivery and On-Demand/ Cloud-Based (SaaS) Delivery); End-Users (Pharmaceutical and Biotechnology Companies, Contract Research Organizations, Business Process Outsourcing Firms, and Others); and Region/Country
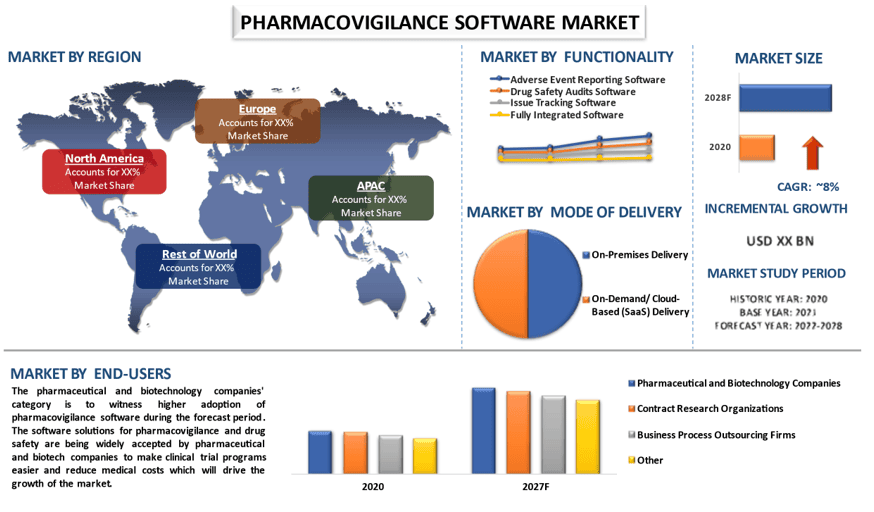
The global pharmacovigilance software market is expected to grow at a significant rate of around 8% during the forecast period. Pharmacovigilance is useful in the early detection and spontaneous reporting of adverse drug reactions, and it is the process of monitoring and evaluating adverse drug reactions and it is a key component of effective drug regulation systems, public health programs and clinical research programs. To meet the requirements of customers, vendors of pharmacovigilance software offer individualized and complete solutions. These vendors are additionally providing end-to-end solutions, for example, electronic information catch, artificial intelligence, and interoperability, among others to enhance their offerings. In addition, stakeholders in the industry are moving toward the use of modern technology to help them with their pharmacovigilance tasks because of the combined pressures of volume, cost, and the increased demand for analytics.
Changing reporting systems and emerging technology in pharmacovigilance and rising cancer cases boosting the R&D and clinical research programs are driving the market growth. For instance, according to the data published by the American Cancer Society (ACS), in January 2020, around 1.8 million new cancer cases were diagnosed and 606,520 cancer deaths in the United States.
IQVIA Inc., Labcorp Drug Development, Parexel International Corporation, Accenture, Cognizant, Clario, Thermo Fisher Scientific Inc., ICON plc, Quanticate, and AB Cube S.A.S. are some of the key players in the market. Several M&As along with partnerships have been undertaken by these players to facilitate customers with hi-tech and innovative products/technologies.
Insights Presented in the Report
“Amongst functionality, adverse event reporting software category to witness robust CAGR during the forecast period”
Based on functionality, the market is segmented into adverse event reporting software, drug safety audits software, issue tracking software, and fully integrated software. Amongst, the adverse event reporting software category is to witness robust CAGR during the forecast period owing to the rising demand for safe medicines and drugs around the world on account of the increasing incidences of adverse drug reactions (ADRs) is contributing to market growth. Apart from this, due to the rapid digitization of clinical trials, there’s a rise in the adoption of pharmacovigilance and medicine safety software to ensure the implementation of new methods also drives the market growth during the forecast period.
“Amongst end-users, the pharmaceutical and biotechnology companies to hold a significant share in the market in 2020”
On the basis of end-users, the market is categorized into pharmaceutical and biotechnology companies, contract research organizations, business process outsourcing firms, and others. Among these, the pharmaceutical and biotechnology companies to hold a significant share of the market in 2020. This is mainly due to the extensive research and development efforts by several pharmaceutical companies and these companies are also launching new products which will also contribute to the growth of the market. For instance, Dialog Solutions launched the next generation of pharmacovigilance literature monitoring in 2021.
In addition, multiple adverse events, or adverse reactions force research organizations to turn to pharmacovigilance services for clinical trials, raising the demand for the growth of the market during the forecast period.
“North America to hold a significant share in the market”
For a better understanding of the market adoption of the pharmacovigilance software industry, the market is analyzed based on its worldwide presence in the countries such as North America (U.S., Canada, Rest of North America), Europe (Germany, U.K., France, Spain, Italy, Rest of Europe), Asia-Pacific (China, Japan, India, Rest of Asia-Pacific), Rest of World. North America is anticipated to grow at a substantial CAGR during the forecast period. This is mainly due to increasing Initiatives undertaken by the governments, investment in the research & development process and adoption of solutions, and the availability of major key market players in the region propelling the growth rate of the market growth during the forecast period. For instance, in 2021, the Open FDA initiative undertaken by the US government provides access to its database through open search-based programs for application developers and scientists.
Reasons to buy this report:
- The study includes market sizing and forecasting analysis validated by authenticated key industry experts.
- The report presents a quick review of overall industry performance at one glance.
- The report covers an in-depth analysis of prominent industry peers with a primary focus on key business financials, product portfolio, expansion strategies, and recent developments.
- Detailed examination of drivers, restraints, key trends, and opportunities prevailing in the industry.
- The study comprehensively covers the market across different segments.
- Deep dive regional level analysis of the industry.
Customization Options:
The global pharmacovigilance software market can further be customized as per the requirement or any other market segment. Besides this, UMI understands that you may have your own business needs, hence feel free to connect with us to get a report that completely suits your requirements.
Table of Content
Research Methodology for the Pharmacovigilance Software Market Analysis (2022-2028)
Analyzing the historical market, estimating the current market, and forecasting the future market of the global pharmacovigilance software market were the three major steps undertaken to create and analyze the adoption of the pharmacovigilance software market in major regions globally. Exhaustive secondary research was conducted to collect the historical market numbers and estimate the current market size. Secondly, to validate these insights, numerous findings and assumptions were taken into consideration. Moreover, exhaustive primary interviews were also conducted, with industry experts across the value chain of the global pharmacovigilance software market. Post assumption and validation of market numbers through primary interviews, we employed a top-down/bottom-up approach to forecasting the complete market size. Thereafter, market breakdown and data triangulation methods were adopted to estimate and analyze the market size of segments and sub-segments of the industry pertains to. Detailed methodology is explained below:
Analysis of Historical Market Size
Step 1: In-Depth Study of Secondary Sources:
Detail secondary study was conducted to obtain the historical market size of the pharmacovigilance software market through company internal sources such as annual reports & financial statements, performance presentations, press releases, etc., and external sources including journals, news & articles, government publications, competitor publications, sector reports, third-party database, and other credible publications.
Step 2: Market Segmentation:
After obtaining the historical market size of the pharmacovigilance software market, we conducted a detailed secondary analysis to gather historical market insights and share for different segments & sub-segments for major regions. Major segments are included in the report as functionality, mode of delivery, and end-users. Further country-level analyses were conducted to evaluate the overall adoption of testing models in that region.
Step 3: Factor Analysis:
After acquiring the historical market size of different segments and sub-segments, we conducted a detailed factor analysis to estimate the current market size of the Pharmacovigilance Software Market. Further, we conducted factor analysis using dependent and independent variables such as various functionality, mode of delivery, and end-users of the pharmacovigilance software market. A thorough analysis was conducted for demand and supply-side scenarios considering top partnerships, mergers and acquisitions, business expansion, and product launches in the pharmacovigilance software market sector across the globe.
Current Market Size Estimate & Forecast
Current Market Sizing: Based on actionable insights from the above 3 steps, we arrived at the current market size, key players in the global pharmacovigilance software market, and market shares of the segments. All the required percentage shares split, and market breakdowns were determined using the above-mentioned secondary approach and were verified through primary interviews.
Estimation & Forecasting: For market estimation and forecast, weights were assigned to different factors including drivers & trends, restraints, and opportunities available for the stakeholders. After analyzing these factors, relevant forecasting techniques i.e., the top-down/bottom-up approach were applied to arrive at the market forecast for 2028 for different segments and sub-segments across the major markets globally. The research methodology adopted to estimate the market size encompasses:
- The industry’s market size, in terms of revenue (USD) and the adoption rate of the pharmacovigilance software market across the major markets domestically
- All percentage shares, splits, and breakdowns of market segments and sub-segments
- Key players in the global pharmacovigilance software market in terms of products offered. Also, the growth strategies adopted by these players to compete in the fast-growing market
Market Size and Share Validation
Primary Research: In-depth interviews were conducted with the Key Opinion Leaders (KOLs) including Top Level Executives (CXO/VPs, Sales Head, Marketing Head, Operational Head, Regional Head, Country Head, etc.) across major regions. Primary research findings were then summarized, and statistical analysis was performed to prove the stated hypothesis. Inputs from primary research were consolidated with secondary findings, hence turning information into actionable insights.
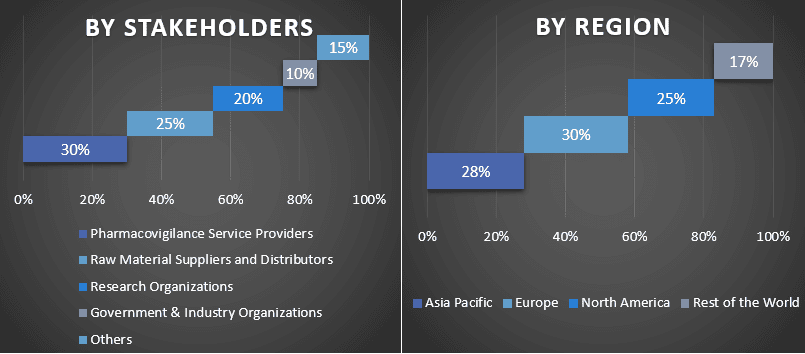
Split of Primary Participants in Different Regions
Market Engineering
The data triangulation technique was employed to complete the overall market estimation and to arrive at precise statistical numbers for each segment and sub-segment of the global pharmacovigilance software market. Data was split into several segments & sub-segments post studying various parameters and trends in the areas of functionality, mode of delivery, and end-users in the global pharmacovigilance software market.
The main objective of the Global Pharmacovigilance Software Market Study
The current & future market trends of the global pharmacovigilance software market were pinpointed in the study. Investors can gain strategic insights to base their discretion for investments on the qualitative and quantitative analysis performed in the study. Current and future market trends determined the overall attractiveness of the market at a regional level, providing a platform for the industrial participant to exploit the untapped market to benefit from a first-mover advantage. Other quantitative goals of the studies include:
- Analyze the current and forecast market size of the pharmacovigilance software market in terms of value (USD). Also, analyze the current and forecast market size of different segments and sub-segments
- Segments in the study include areas of functionality, mode of delivery, and end-users.
- Define and analysis of the regulatory framework for the pharmacovigilance software industry.
- Analyze the value chain involved with the presence of various intermediaries, along with analyzing customer and competitor behaviors of the industry.
- Analyze the current and forecast market size of the pharmacovigilance software market for the major region.
- Major countries of regions studied in the report include Asia Pacific, Europe, North America, and the Rest of the World.
- Company profiles of the Pharmacovigilance Software Market and the growth strategies adopted by the market players to sustain in the fast-growing market
- Deep dive regional level analysis of the industry
Related Reports
Customers who bought this item also bought