- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
Southeast Asia Prefilled Syringes Market: Current Analysis and Forecast (2025-2033)
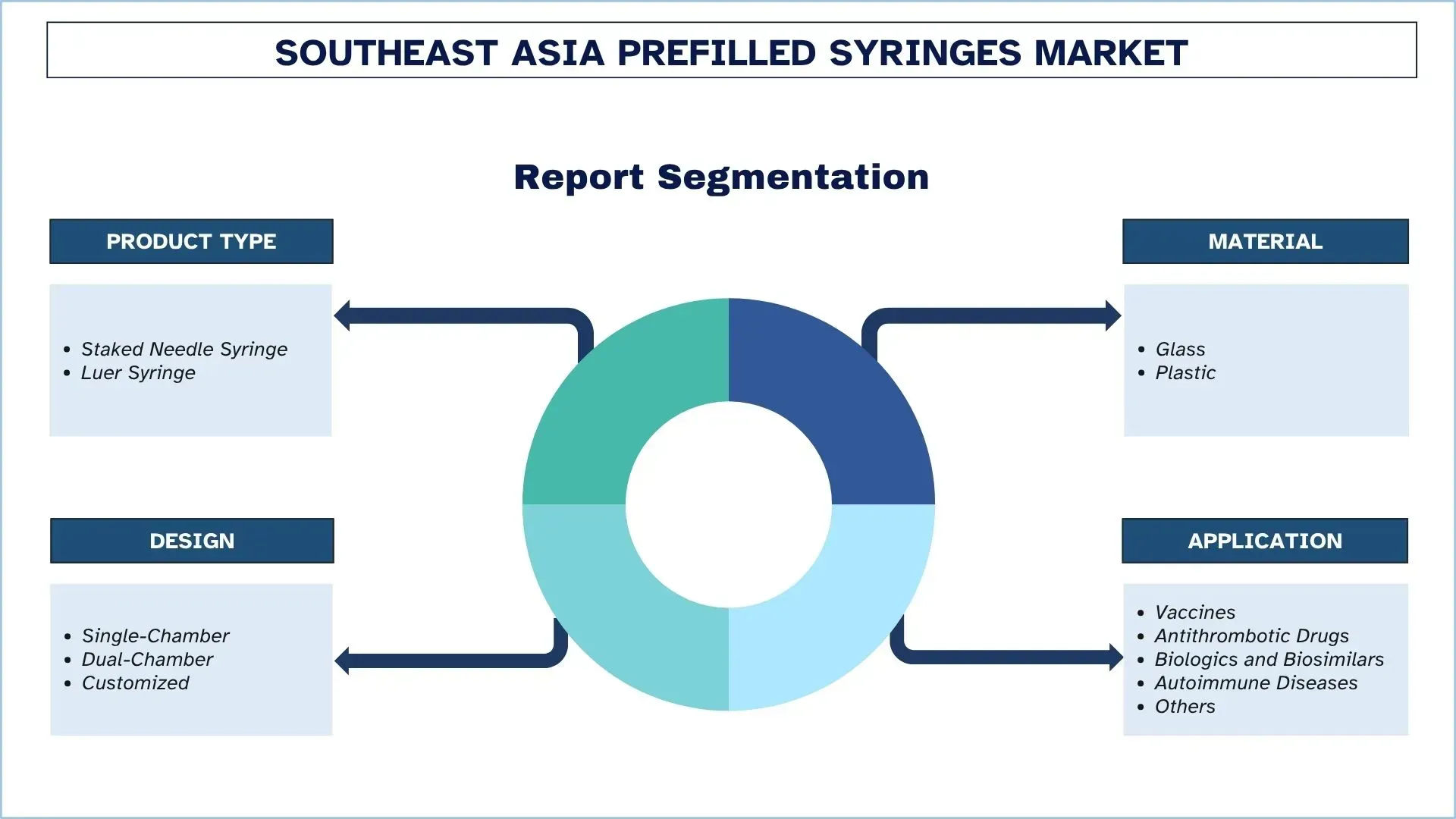
Emphasis on Product Type (Staked Needle Syringe, Luer Syringe); Material (Glass, Plastic); Design (Single-Chamber, Dual-Chamber, Customized); Application (Vaccines, Antithrombotic Drugs, Biologics and Biosimilars, Autoimmune Diseases, Others); and Country.
Southeast Asia Prefilled Syringes Market Size & Forecast
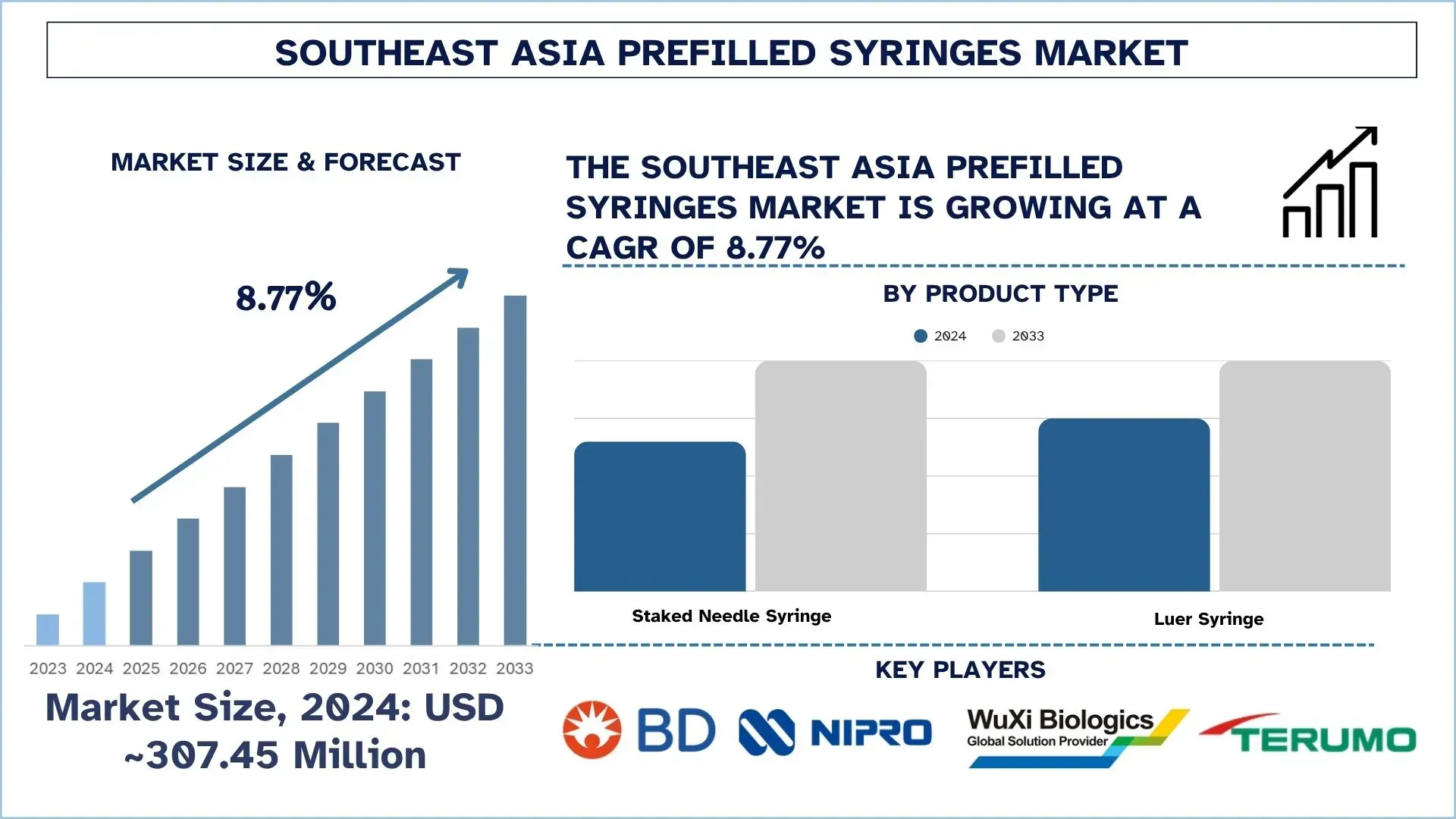
The Southeast Asia Prefilled Syringes Market was valued at ~USD 307.45 million in 2024 and is expected to grow at a strong CAGR of approximately 8.77% during the forecast period (2025-2033F), driven by an increased production and use of biologics, biosimilars, and large-scale immunization programs, which are fueling prefilled syringe demand.
Southeast Asia Prefilled Syringes Market Analysis
Prefilled syringes are single-dose, ready-to-use injection devices that are loaded with the preset amount of medication, aimed at improving the accuracy of the dose and safe administration to patients. These are filled with pharmaceutical drugs, and so the chance of contamination is minimal, and they are used with ease of administration in both clinical and domestic care settings.
In Southeast Asia, pharmaceutical companies and healthcare providers are moving towards the automation of fill-finish processes, the use of polymer-based syringes to deliver biologics, and high-tech sterilization processes to meet the increasing quality requirements. To reduce reliance on imports, companies are emphasizing cleanroom growth, contract manufacturing agreements, and localized inner-city syringe assembly. Moreover, increased interest in patient-centric drug delivery systems and self-injection systems has also facilitated the development of investments in safety-designed and customizable syringe forms. These developments are being encouraged by governments and private healthcare networks through the provision of better regulatory frameworks, vaccination efforts, and the encouragement of local manufacturing of medical devices.
On July 29, 2025, WuXi Biologics announced construction had begun for a new modular Drug Product (DP) facility, which will become part of the company’s CRDMO hub in Singapore. Under a strategic collaboration between WuXi Biologics and Pharmadule Morimatsu, 470 modular components are being fabricated at Morimatsu’s plant in Changshu City and will be transported to Singapore’s Tuas Biomedical Park for installation. Once complete, the building will be one of the world’s largest modular biologics DP facilities, comprising approximately 30,000 square meters of space.
The new facility will feature state-of-the-art manufacturing lines, centralized Quality Control (QC) labs supporting full release and stability testing, and Manufacturing Science and Technology (MSAT) labs, significantly enhancing the company’s end-to-end DP services capabilities. Equipped with three pre-filled syringe (PFS) production lines and two vial production lines for both liquid and lyophilized products.
Southeast Asia Prefilled Syringes Market Trends
This section discusses the key market trends that are influencing the various segments of the Southeast Asia Prefilled Syringes market, as found by our team of research experts.
Shift Toward Plastic Prefilled Syringes
The adoption of plastic prefilled syringes is emerging as a major trend in the Southeast Asia market, driven by the increasing demand for lightweight, durable, and shatter-resistant alternatives to glass. Plastic syringes, especially those that are composed of cyclic olefin polymer (COP) and cyclic olefin copolymer (COC), are more chemically and biologically compatible and resistant to sensitive drugs and biologics. The reduced risk associated with breakage qualifies them as suitable for mass immunization programs and for self-administration. With the modernization of healthcare systems in the region, manufacturers are investing in new technologies in plastic molding and barrier coating. Therefore, the rising shift toward increased patient safety aligns with the sustainability and cost-efficiency objectives of pharmaceutical packaging.
Southeast Asia Prefilled Syringes Industry Segmentation
This section provides an analysis of the key trends in each segment of the Southeast Asia Prefilled Syringes market, along with forecasts at the country level for 2025-2033.
The staked needle prefilled syringes market held the dominant share of the Prefilled Syringes market in 2024.
Based on product type, the market is segmented into staked needle syringe and luer syringe. Among these, the staked needle prefilled syringes market held the largest share in 2024. This is mainly due to its ability to provide ready-to-use, contamination-free solutions that are applicable in mass immunization and chronic care therapies. The integrated design saves on assembly time and ensures the correct dosage is administered, making it very appealing to large-scale healthcare programs. Moreover, with the growth of regional production, producers are embracing automation and safety improvements in order to fulfill the increasing demand in an efficient manner.
The dual-chamber prefilled syringes market is expected to grow at a significant CAGR during the forecast period (2025-2033).
Based on design, the market is segmented into single-chamber, dual-chamber, and customized. Among these, the dual-chamber prefilled syringes market is expected to grow at a significant CAGR during the forecast period (2025-2033). This is mainly due to the increasing trend of dual-chamber prefilled syringe innovation, as biologics and lyophilized drugs require careful reconstitution before injection. This segment helps differentiate the products by incorporating the drug and diluent into a single device, thereby providing patients with convenience. With the growing adoption of combination therapies, companies are investing in sophisticated dual-chamber designs to increase their high-value injectable lines.
Singapore held a dominant share of the Southeast Asian Prefilled Syringes market in 2024
Singapore is the technological and regulatory support of the Southeast Asia prefilled syringes market. It also features world-class sterile manufacturing and fill-finish facilities, making it an ideal destination for regional and global pharmaceutical companies. The stringent quality in the country and the open system of regulations promote innovation and massive biological filling. The high-cleanroom automation, safety syringes design, and R&D partnerships continue to make the production efficient. The government of Singapore is actively involved in financing and collaborating with medical technology and making it a hub for injectable drug delivery. In addition to consolidating the domestic manufacturing, this leadership also contributes to the knowledge transfer and the reliability of supply chains throughout Southeast Asia.
For instance, on May 19, 2023, Thermo Fisher Scientific, the world leader in serving science, opened a new sterile drug facility in Singapore that will better enable customers to deliver new medicines and vaccines in the Asia-Pacific market. The new facility also marks a significant milestone and investment in pandemic preparedness for Singapore, which is fast emerging as a biomedical hub in the Asia-Pacific region.
Southeast Asia Prefilled Syringes Industry Competitive Landscape
The Southeast Asia Prefilled Syringes market is competitive, with several global and international market players. The key players are adopting different growth strategies to enhance their market presence, such as partnerships, agreements, collaborations, new product launches, geographical expansions, and mergers and acquisitions.
Top Southeast Asia Prefilled Syringes Companies
Some of the major players in the market are Becton Dickinson Holdings Pte Ltd (BD), NIPRO Corporation, WuXi Biologics, Terumo Corporation, SCHOTT Pharma (SCHOTT AG), Sandoz Singapore Pte. Ltd. (Sandoz Group AG), West Pharmaceutical Services, Inc., Weigao Medical International Co., Ltd (Weigao Group), Gerresheimer AG, PT Etana Biotechnologies Indonesia.
Recent Developments in the Southeast Asia Prefilled Syringes Market
On April 24, 2025, SCG Packaging Public Company Limited, also known as SCGP, announced that the company is implementing a strategy to enhance its capabilities in the growing healthcare supplies market, which continues to show strong growth potential. This involves entering the syringes and needles market. The company’s board of directors approved an investment project to expand syringes production capacity by 180 million units per year and needles production by 100 million units per year at VEM (Thailand) Company Limited (VEM-TH) in SCGP, Rayong Province, with a total investment of Baht 142.3 million (~USD 4.37 million). Production is expected to begin in January 2026.
On February 2, 2023, Pharmaniaga Bhd’s (Pharmaniaga) wholly-owned subsidiary, Pharmaniaga LifeScience Sdn Bhd (PLSB), successfully installed a pre-filled syringe (PFS) filling line - the first of its kind in Malaysia. The line is part of PLSB’s ongoing expansion plan to become the world’s first halal vaccine manufacturing plant. Additionally, the plant has commissioned other PFS packing equipment, including a de-nester, an automatic visual inspection (AVI) machine, a plunger insertion machine, a labelling unit, and a two-in-one combination unit of washer-steriliser.
Southeast Asia Prefilled Syringes Market Report Coverage
Report Attribute | Details |
Base year | 2024 |
Forecast period | 2025-2033 |
Growth momentum | Accelerate at a CAGR of 8.77% |
Market size 2024 | ~USD 307.45 million |
Country analysis | Indonesia, Thailand, Philippines, Vietnam, Malaysia, Singapore, Rest of Southeast Asia |
Major contributing Country | Vietnam is expected to grow at the highest CAGR during the forecasted period. |
Companies profiled | Becton Dickinson Holdings Pte Ltd (BD), NIPRO Corporation, WuXi Biologics, Terumo Corporation, SCHOTT Pharma (SCHOTT AG), Sandoz Singapore Pte. Ltd. (Sandoz Group AG), West Pharmaceutical Services, Inc., Weigao Medical International Co., Ltd (Weigao Group), Gerresheimer AG, PT Etana Biotechnologies Indonesia. |
Report Scope | Market Trends, Drivers, and Restraints; Revenue Estimation and Forecast; Segmentation Analysis; Demand and Supply Side Analysis; Competitive Landscape; Company Profiling |
Segments Covered | By Product Type, By Material, By Design, By Application, By Country |
Reasons to Buy the Southeast Asia Prefilled Syringes Market Report:
The study includes market sizing and forecasting analysis confirmed by authenticated key industry experts.
The report briefly reviews overall industry performance at a glance.
The report covers an in-depth analysis of prominent industry peers, primarily focusing on key business financials, product portfolios, expansion strategies, and recent developments.
Detailed examination of drivers, restraints, key trends, and opportunities prevailing in the industry.
The study comprehensively covers the market across different segments.
Customization Options:
The Southeast Asia Prefilled Syringes market can further be customized as per requirements or any other market segment. Besides this, UnivDatos understands that you may have your own business needs; hence, feel free to contact us to get a report that completely suits your requirements.
Table of Content
Research Methodology for the Southeast Asia Prefilled Syringes Market Analysis (2023-2033)
We analyzed the historical market, estimated the current market, and forecasted the future market of the Southeast Asian Prefilled Syringes market to assess its application in major countries. We conducted exhaustive secondary research to gather historical market data and estimate the current market size. To validate these insights, we carefully reviewed numerous findings and assumptions. Additionally, we conducted in-depth primary interviews with industry experts across the Southeast Asian Prefilled Syringes value chain. After validating market figures through these interviews, we used both top-down and bottom-up approaches to forecast the overall market size. We then employed market breakdown and data triangulation methods to estimate and analyze the market size of industry segments and sub-segments.
Market Engineering
We employed the data triangulation technique to finalize the overall market estimation and derive precise statistical numbers for each segment and sub-segment of the Southeast Asia Prefilled Syringes market. We split the data into several segments and sub-segments by analyzing various parameters and trends, including product type, material, design, application, and country within the Southeast Asian Prefilled Syringes market.
The Main Objective of the Southeast Asia Prefilled Syringes Market Study
The study identifies current and future trends in the Southeast Asia Prefilled Syringes market, providing strategic insights for investors. It highlights market attractiveness, enabling industry participants to tap into untapped markets and gain a first-mover advantage. Other quantitative goals of the studies include:
Market Size Analysis: Assess the current and forecast market size of the Southeast Asia Prefilled Syringes market and its segments in terms of value (USD).
Southeast Asia Prefilled Syringes Market Segmentation: Segments in the study include areas of product type, material, design, application, and country.
Regulatory Framework & Value Chain Analysis: Examine the regulatory framework, value chain, customer behavior, and competitive landscape of the Southeast Asia Prefilled Syringes industry.
Country Analysis: Conduct a detailed country analysis for key areas such as Indonesia, Thailand, the Philippines, Vietnam, Malaysia, Singapore, and the Rest of Southeast Asia.
Company Profiles & Growth Strategies: Company profiles of the Southeast Asia Prefilled Syringes market and the growth strategies adopted by the market players to sustain in the fast-growing market.
Frequently Asked Questions FAQs
Q1: What is the Southeast Asia Prefilled Syringes market’s current market size and growth potential?
The Southeast Asia Prefilled Syringes market was valued at ~USD 307.45 million in 2024 and is projected to expand at a CAGR of 8.77% from 2025 to 2033. This strong growth is fueled by the rising demand for biologics, increased vaccination programs, and the shift toward safer, ready-to-use injectable solutions across healthcare systems in Singapore, Indonesia, and Vietnam.
Q2: Which segment has the largest share of the Southeast Asia Prefilled Syringes market by product type?
The Staked Needle Syringe segment holds the largest market share in Southeast Asia due to its widespread use in vaccines and chronic disease treatments, ease of administration, and growing preference in hospital and self-injection settings.
Q3: What are the driving factors for the growth of the Southeast Asia Prefilled Syringes market?
Growth is driven by the rising prevalence of chronic diseases, the surge in biologics and biosimilars, government-led vaccination drives, and expanding investments by global syringe manufacturers to establish local production facilities in Southeast Asia.
Q4: What are the emerging technologies and trends in the Southeast Asia Prefilled Syringes market?
Key trends include a shift from glass to plastic syringes for improved safety, adoption of dual-chamber and customized designs, integration of smart and eco-friendly syringe materials, and rising automation across regional manufacturing plants to enhance precision and reduce contamination risks.
Q5: What are the key challenges in the Southeast Asia Prefilled Syringes market?
Major challenges include high manufacturing and sterilization costs, complex regulatory frameworks across ASEAN countries, limited cold-chain infrastructure for biologics, and dependence on imported components for syringe production.
Q6: Which country dominates the Southeast Asia Prefilled Syringes market?
Singapore leads the regional market, supported by strong pharmaceutical infrastructure, advanced biologics manufacturing capabilities, and early adoption of prefilled syringe technologies for vaccine and biologic drug delivery.
Q7: Who are the key players in the Southeast Asia Prefilled Syringes market?
Leading companies in the Southeast Asia Prefilled Syringes market include:
• Becton Dickinson Holdings Pte Ltd (BD)
• NIPRO Corporation
• WuXi Biologics
• Terumo Corporation
• SCHOTT Pharma (SCHOTT AG)
• Sandoz Singapore Pte. Ltd. (Sandoz Group AG)
• West Pharmaceutical Services, Inc.
• Weigao Medical International Co., Ltd (Weigao Group)
• Gerresheimer AG
• PT Etana Biotechnologies Indonesia
Q8: What investment opportunities exist in the Southeast Asia Prefilled Syringes market?
Investors can find strong opportunities in local manufacturing expansion, syringe material innovation, and biologic fill-finish partnerships. The region’s growing pharmaceutical base and supportive government healthcare initiatives make it attractive for long-term investment and strategic collaborations.
Q9: How is technology influencing the development of prefilled syringes in Southeast Asia?
Technologies such as AI-based quality control, advanced polymer materials (COP/COC), and smart labeling for traceability are revolutionizing production efficiency and safety standards, driving faster adoption across hospitals and biotech companies
Related Reports
Customers who bought this item also bought