- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
Duchenne Muscular Dystrophy Market Insight, Epidemiology & Forecast (2024-2032)
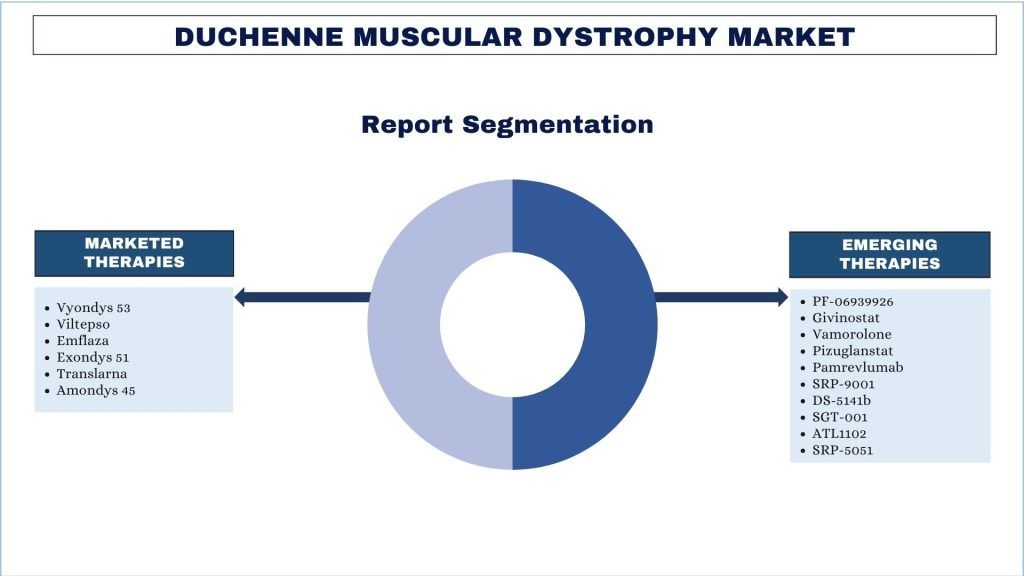
Emphasis on Marketed Therapies (Vyondys 53, Viltepso, Emflaza, Exondys 51, Translarna, and Amondys 45); Emerging Therapies (PF-06939926, Givinostat, Vamorolone, Pizuglanstat (TAS-205), Pamrevlumab (FG-3019), SRP-9001, Allogeneic Cardiosphere-derived Cells (CAP-1002), DS-5141b, SGT-001, ATL1102, SRP-5051) and Country
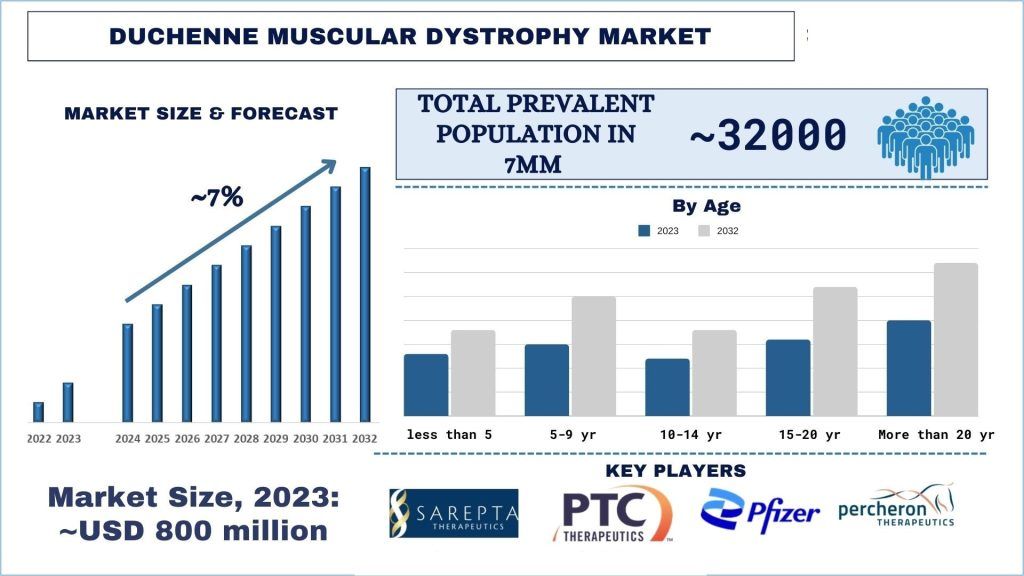
Duchenne Muscular Dystrophy Market Size & Forecast
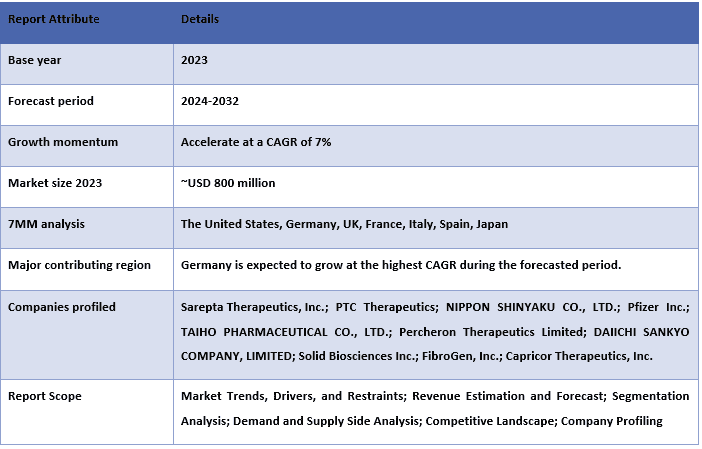
The Duchenne Muscular Dystrophy Market was valued at approximately USD 800 million in 2023 and is expected to grow at a strong CAGR of around ~7% during the forecast period (2024-2032) owing to the robust pipeline activity, advancements in genetic research, and patient-centered drug development.
Duchenne Muscular Dystrophy Market Analysis
Duchenne Muscular Dystrophy (DMD) is a rare genetic disorder characterized by progressive muscle weakness and degeneration. It primarily affects males, with symptoms typically appearing in early childhood. DMD is caused by mutations in the DMD gene, which encodes the protein dystrophin, essential for maintaining muscle integrity. Without functional dystrophin, muscle fibers become damaged and eventually replaced by fibrotic and fatty tissue, leading to loss of mobility, respiratory complications, and cardiac issues.
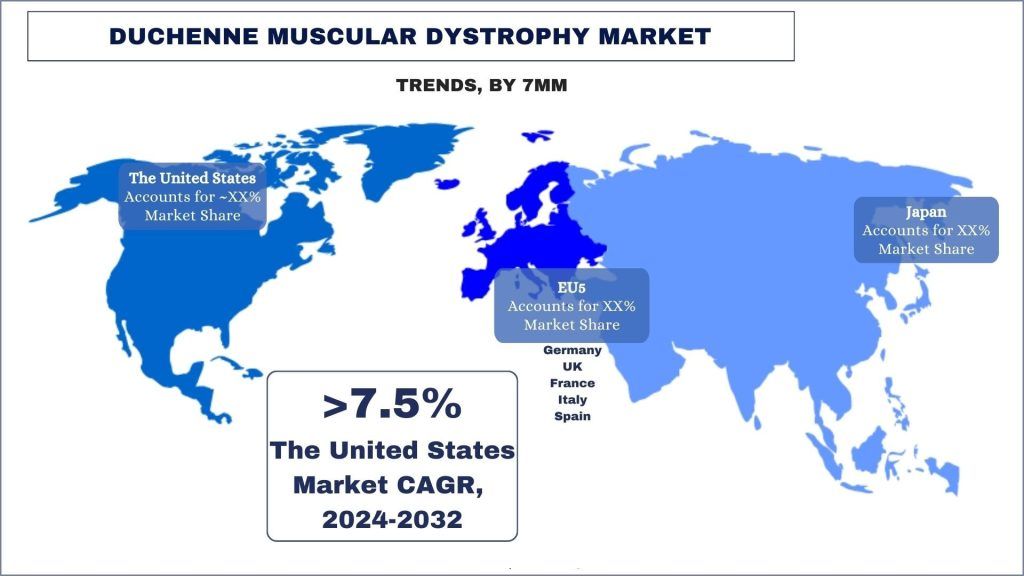
In the 7MM (Seven Major Markets) – the United States, European Union Five (EU5: France, Germany, Italy, Spain, United Kingdom), and Japan – DMD prevalence varies but is estimated to affect approximately 1 in 3,500 male births globally in 2024. Despite its rare occurrence, DMD imposes a significant burden on patients, families, and healthcare systems due to its debilitating nature and lifelong management requirements. While advances in treatment and supportive care have improved patients’ outcomes and quality of life, there remains an unmet need for disease-modifying therapies capable of halting or reversing disease progression.
Duchenne Muscular Dystrophy: Disease Overview
Cause: Genetic Mutation: – DMD is caused by mutations in the dystrophin gene, which provides instructions for producing the protein dystrophin. Dystrophin is crucial for maintaining the structure and function of muscle fibers.
Inheritance: DMD is an X-linked recessive disorder primarily affecting males. Females can carry the genetic mutation without showing symptoms but may pass it on to their children.
In Duchenne Muscular Dystrophy, adoption, and implementation efforts have led to significant disease management and patient care advancements. Regulatory approvals for innovative therapies, such as exon-skipping drugs and gene therapies, provided patients with access to treatments targeting the underlying genetic causes of the disease. For instance, on June 22, 2023, the U.S. Food and Drug Administration approved Elevidys, the first gene therapy for the treatment of pediatric patients 4 through 5 years of age with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene who do not have a pre-existing medical reason preventing treatment with this therapy.
Moreover, telemedicine platforms and remote monitoring technologies enable DMD patients to access specialized care, consultation, and monitoring remotely.
Duchenne Muscular Dystrophy: Diagnosis
Duchenne Muscular Dystrophy (DMD) diagnosis typically begins with the observation of clinical symptoms, often in early childhood, such as difficulty walking, frequent falls, and muscle weakness. These symptoms usually appear between the ages of 2 and 5 years. Elevated levels of creatine kinase (CK) in the blood, often 10 to 100 times higher than normal, are a common initial indicator of DMD. Genetic testing confirms the diagnosis by identifying mutations in the DMD gene on the X chromosome, responsible for producing the protein dystrophin.
According to the National Institute of Health, in 2024, approximately 1 in every 3,500 to 5,000 male births worldwide is affected by DMD. Muscle biopsy, though less commonly used due to the effectiveness of genetic testing, can also reveal the absence or deficiency of dystrophin. Early and accurate diagnosis is crucial for managing DMD and improving the quality of life for affected individuals, as it allows for timely intervention with therapies and supportive care.
Duchenne Muscular Dystrophy: Treatment
Duchenne Muscular Dystrophy (DMD) is a progressive and currently incurable genetic disorder, but several treatments and therapies can help manage symptoms, improve quality of life, and slow disease progression.
First-Line Treatment—Corticosteroids, particularly prednisone and deflazacort, are considered first-line treatments for Duchenne Muscular Dystrophy (DMD) to help manage symptoms and slow disease progression. These medications reduce inflammation and prolong muscle function in individuals with DMD. Prednisone and deflazacort have been shown to improve muscle strength, delay the loss of ambulation, and extend survival in people with DMD.
In the market, prednisone and deflazacort are available in various formulations, including tablets and oral suspension, to accommodate different age groups and dosage requirements. Both medications have been approved for the treatment of DMD in several countries.
The other treatments are Exon Skipping Therapy, Gene Therapy, Cardiac Care, Respiratory Care, Physical Therapy and Exercise, Orthopedic Interventions, Nutritional Support, Psychological and Social Support, Research and Clinical Trials.
Duchenne Muscular Dystrophy: Therapeutic Strategies
Corticosteroids: prednisone and deflazacort are available in various formulations, including tablets and oral suspension, to accommodate different age groups and dosage requirements. Both medications have been approved for the treatment of DMD in several countries.
Exon Skipping Therapy: This includes treatments like Eteplirsen (Exondys 51), which aims to skip over faulty parts of the DMD gene to produce a functional dystrophin protein.
Gene Therapy: Experimental approaches such as microdystrophin gene therapy are designed to deliver a functional version of the dystrophin gene to muscle cells.
Physical Therapy and Exercise: Regular stretching, strengthening exercises, and the use of assistive devices help maintain muscle function, flexibility, and mobility.
Duchenne Muscular Dystrophy: Epidemiology
The report on Duchenne Muscular Dystrophy epidemiology includes both historical and forecasted data, segmented by various parameters. It covers:
- Total Prevalent Population of Duchenne Muscular Dystrophy
- Age-specific Prevalent Population of Duchenne Muscular Dystrophy
- Ambulatory and Non-ambulatory Population of Duchenne Muscular Dystrophy
- Mutation-specific Prevalent Population of Duchenne Muscular Dystrophy
The analysis spans the 7MM market, including the United States, EU5 countries (Germany, France, Italy, Spain, and the United Kingdom), and Japan, from 2024 to 2032.
Major Insights:
This section provides an overview of the Duchenne Muscular Dystrophy (DMD) epidemiology across the 7MM regions.
In 2023, the total prevalent population of DMD in the 7MM was ~30,000-35,000. The epidemiological assessment for DMD revealed that about 250,000 people in the United States have some form of muscular dystrophy.
Within the EU-5 countries, the United Kingdom had the highest number of prevalent DMD cases in 2023; about 100 boys are born with Duchenne MD each year. In Germany, the prevalence of DMD is as low as 1.5 per 100,000.
In the United States in 2023, the highest age-specific prevalence of DMD was observed in the 5-9 years age group, followed by the 10-14 years age group.
In 2021, around 60–70% of mutations in patients with DMD are deletions.
In 2021, the prevalence of DMD is 1.9 to 3.4 per 100,000 individuals, and there are an estimated 2,500 to 4,000 patients in Japan.
The United States holds the dominant share of the market in 2023.
Duchenne Muscular Dystrophy (DMD) is a genetic neuromuscular disorder characterized by the progressive degeneration of muscle tissue. It primarily affects males and is caused by mutations in the dystrophin gene located on the X chromosome. Advances in diagnostic techniques and increased awareness among healthcare providers and the public have led to earlier and more accurate detection of DMD cases. Moreover, improvements in supportive care and medical interventions have extended the lifespan of individuals with DMD, contributing to a larger identified population living with the condition. Additionally, ongoing research efforts and the development of novel therapies offer hope for improved outcomes and quality of life for DMD patients in the United States.
In 2023, the total dystrophy disorders affect anywhere between 1 in 5,000 to 1 in 6,000 people assigned male at birth. About 250,000 people in the United States have some form of muscular dystrophy. Experts estimate fewer than 50,000 people in the United States have DMD.
Duchenne Muscular Dystrophy: Drug Profile
The Duchenne Muscular Dystrophy (DMD) Drug Profile provides a comprehensive overview of the therapeutic landscape for DMD. This section covers the various treatment options available, including current standard-of-care therapies, emerging drugs in the pipeline, and innovative treatments under development. Each section of drugs delves into the mechanisms of action, clinical trial progress, regulatory status, and potential impact of these drugs on the management of DMD. By exploring both existing and upcoming therapies, this section aims to offer a detailed understanding of the evolving treatment paradigm for Duchenne Muscular Dystrophy.
Duchenne Muscular Dystrophy: Marketed Therapies
Vyondys 53: Sarepta Therapeutics
Description
VYONDYS 53 is an FDA-approved Duchenne muscular dystrophy treatment for patients who have a confirmed genetic mutation in the dystrophin gene that can be treated by skipping exon 53. In some patients, it helps the body make a shorter form of the dystrophin protein. Golodirsen was developed by Sarepta Therapeutics and granted accelerated FDA approval on December 12, 2019, due to the urgent need for this drug in patients suffering from a certain form of DMD. Golodirsen is a morpholino antisense oligomer designed to treat about 8% of patients with Duchenne Muscular Dystrophy (DMD).
Detailed Product Information Included in the Report…
Duchenne Muscular Dystrophy Industry Overview
The Duchenne Muscular Dystrophy market is competitive, with several 7MM and international market players. The key players are adopting different growth strategies to enhance their market presence, such as partnerships, agreements, collaborations, new product launches, geographical expansions, and mergers and acquisitions. Some of the major players operating in the market are Sarepta Therapeutics, Inc. ; PTC Therapeutics; NIPPON SHINYAKU CO., LTD.; Pfizer Inc.; TAIHO PHARMACEUTICAL CO., LTD.; Percheron Therapeutics Limited; DAIICHI SANKYO COMPANY, LIMITED; Solid Biosciences Inc.; FibroGen, Inc.; Capricor Therapeutics, Inc.
Duchenne Muscular Dystrophy Market News
In January 2023, Sarepta Therapeutics announced the signing of a commercial supply agreement for Catalent to manufacture delandistrogene moxeparvovec (SRP-9001), Sarepta’s most advanced gene therapy candidate for the treatment of Duchenne muscular dystrophy (DMD).
Duchenne Muscular Dystrophy Market Report Coverage
Reasons to buy this report:
- The study includes market sizing and forecasting analysis validated by authenticated key industry experts.
- The report presents a quick review of overall industry performance at one glance.
- The report covers an in-depth analysis of prominent industry peers with a primary focus on key business financials, product portfolios, expansion strategies, and recent developments.
- Detailed examination of drivers, restraints, key trends, and opportunities prevailing in the industry.
- The study comprehensively covers the market across different segments.
- Deep dive regional level analysis of the industry.
Customization Options:
The global Duchenne Muscular Dystrophy market can further be customized as per the requirement or any other market segment. Besides this, UMI understands that you may have your own business needs, hence feel free to connect with us to get a report that completely suits your requirements.
Table of Content
Research Methodology for the Duchenne Muscular Dystrophy Market – Epidemiology & Market Forecast (2024-32F)
Analyzing the historical market, estimating the current market, and forecasting the future market of the Duchenne Muscular Dystrophy market were the three major steps undertaken to create and analyze the total prevalent population of Duchenne Muscular Dystrophy and adoption of Duchenne Muscular Dystrophy therapeutics in 7 Major Markets. Exhaustive secondary research was conducted to collect the historical market numbers and prevalence of diseases and estimate the current market size. Secondly, to validate these insights, numerous findings and assumptions were taken into consideration. Moreover, exhaustive primary interviews were also conducted with industry experts across the value chain of the global Duchenne Muscular Dystrophy market. The assumption and validation of market numbers through primary interviews, we employed a top-down/bottom-up approach to forecasting the patient population and complete market size. Thereafter, market breakdown and data triangulation methods were adopted to estimate and analyze the market size of segments and sub-segments of the industry. Detailed methodology is explained below:
Analysis of Historical Market Size
Step 1: In-Depth Study of Secondary Sources:
An extensive secondary study was conducted to gather data on prevalence/incidence rates, diagnostic rates, treatment rates, historical market sizes, etc., for the Duchenne Muscular Dystrophy market. This involved utilizing internal sources such as annual reports & financial statements, performance presentations, press releases, etc., and external sources including journals, news & articles, government publications, competitor publications, sector reports, third-party database, and other credible publications. This step ensured a robust understanding of the market’s epidemiology and historical context.
Step 2: Exhaustive Epidemiological Investigation
To enhance the accuracy of epidemiological data, we employed a multi-step approach:
Literature Review: An exhaustive review of peer-reviewed journals, clinical trial data, and epidemiological studies to establish baseline prevalence and incidence rates.
Database Analysis: Utilization of specialized databases such as WHO, CDC, and national health registries to corroborate findings and refine prevalence and incidence estimates.
Cross-Validation: Cross-referencing data from multiple sources to ensure consistency and reliability of epidemiological estimates.
Step 3: Market Segmentation:
After obtaining the historical market size of the Duchenne Muscular Dystrophy market, we conducted a detailed secondary analysis to gather historical market insights and share for different segments, sub-segments, and products for major regions. The report includes major segments such as marketed therapies, emerging therapies, and regions. Further country-level analyses were conducted to evaluate the overall adoption of testing models in that region.
Step 4: Factor Analysis:
After acquiring the historical data of different segments and sub-segments, we conducted a detailed factor analysis to estimate the current scenario of the Duchenne Muscular Dystrophy market. Further, we conducted factor analysis using dependent and independent variables such as Marketed Therapies, Emerging Therapies, and country (7MM) of the Duchenne Muscular Dystrophy market. A thorough analysis was conducted for demand and supply-side scenarios considering top partnerships, mergers and acquisitions, business expansion, and product launches in the Duchenne Muscular Dystrophy market sector.
Current Market Size Estimate & Forecast
Current Market Sizing: Based on actionable insights from the above 3 steps, we arrived at the current market size, key players in the Duchenne Muscular Dystrophy market, and market shares of the segments. All the required percentage shares split, and market breakdowns were determined using the above-mentioned secondary approach and were verified through primary interviews.
Estimation & Forecasting: For market estimation and forecast, weights were assigned to different factors including drivers & trends, restraints, and opportunities available for the stakeholders. After analyzing these factors, relevant forecasting techniques i.e., the top-down/bottom-up approach were applied to arrive at the market forecast for 2032 for different segments and sub-segments across the 7 major markets. The research methodology adopted to estimate the market size encompasses:
- The industry’s market size, in terms of revenue (USD) and the prevalent population and available treatments for Duchenne Muscular Dystrophy, and adoption rate of the Duchenne Muscular Dystrophy market across the 7 major markets domestically
- All percentage shares, splits, and breakdowns of market segments and sub-segments
- Key players in the global Duchenne Muscular Dystrophy market in terms of products offered. Also, the growth strategies adopted by these players to compete in the fast-growing market.
Market Size and Share Validation
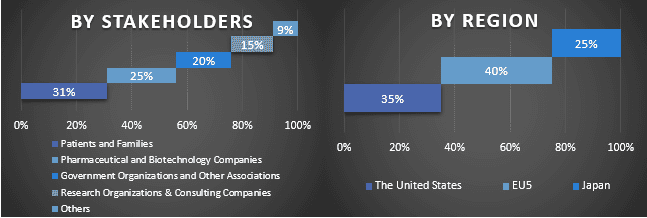
Primary Research: In-depth interviews were conducted with the Key Opinion Leaders (KOLs) including Top Level Executives (CXO/VPs, Sales Head, Marketing Head, Operational Head, Regional Head, Country Head, etc.) across major regions. In Addition to this, interviews with healthcare professionals including physicians, surgeons, and specialist doctors were also conducted to get epidemiology-specific insights. Primary research findings were then summarized, and statistical analysis was performed to prove the stated hypothesis. Inputs from primary research were consolidated with secondary findings, hence turning information into actionable insights.
Split of Primary Participants in Different Regions
Market Engineering
The data triangulation technique was employed to complete the overall market estimation and arrive at precise statistical numbers for each segment and sub-segment of the global Duchenne Muscular Dystrophy market. After studying various parameters and trends in the marketed therapies, emerging therapies, and regions of the global Duchenne Muscular Dystrophy market, data was split into several segments and sub-segments.
The main objective of the Global Duchenne Muscular Dystrophy Market Study
The current & future market trends of the global Duchenne Muscular Dystrophy market were pinpointed in the study. Investors can gain strategic insights to base their discretion for investments on the qualitative and quantitative analysis performed in the study. Current and future market trends determined the overall attractiveness of the market at a regional level, providing a platform for the industrial participant to exploit the untapped market to benefit from a first-mover advantage. Other quantitative goals of the studies include:
- Analyze the current and forecast market size of the Duchenne Muscular Dystrophy market in terms of value (USD). Also, analyze the current and forecast market size of different segments and sub-segments.
- Accurately estimate the prevalence and incidence rates of Duchenne Muscular Dystrophy and understand the diagnostic and treatment rates, aiding in the identification of unmet medical needs and potential market opportunities.
- Segments in the study include areas of the marketed therapies, emerging therapies, and regions.
- Define and analysis of the regulatory framework for the Duchenne Muscular Dystrophy
- Analyze the current and forecast market size of the Duchenne Muscular Dystrophy market for the 7 major Markets.
- Major countries of regions studied in the report include the United States, Germany, the UK, France, Italy, Spain, and Japan.
- Company profiles of the Duchenne Muscular Dystrophy market and the growth strategies adopted by the market players to sustain in the fast-growing market.
- To conduct country-level analyses to evaluate the adoption of testing models and therapies in specific regions.
Frequently Asked Questions FAQs
Q1: What is Duchenne Muscular Dystrophy?
Q2: What is the Duchenne Muscular Dystrophy market's current market size and growth potential?
Q3: What are the driving factors for the growth of the Duchenne Muscular Dystrophy market?
Q4: What is the overall Duchenne Muscular Dystrophy epidemiology scenario in 7MM?
Q5: Which country will dominate the Duchenne Muscular Dystrophy market?
Related Reports
Customers who bought this item also bought