- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
COVID-19 – Pipeline Analysis 2020 for Global Market
Emphasis on Products covered by Phase (Phase III, Phase II, Phase I, Investigational New Drug, Pre-clinical, Discovery); Therapeutic Assessment by Stages (Product Type, Route of Administration, Molecule Type) and Company
COVID-19 (also known as Anderson COVID-19) is a viral disease caused by RNA virus, SARS-CoV-2 or commonly known as corona virus. These viruses can cause respiratory, enteric, hepatic, and neurologic diseases. At the end of 2019, a new coronavirus was identified as the cause of a cluster of pneumonia cases in Wuhan, China. It rapidly spread, resulting in an epidemic throughout China, followed by an increasing number of cases in other countries throughout the world. On March 11, 2020 World Health Organization (WHO) declared it a pandemic.
COVID-19 is mainly transmitted through contact with respiratory droplets rather than through the air and spreads primarily through contact with an infected person when they cough or sneeze. It also spreads when a person touches a surface or object that has the virus on it, then touches their eyes, nose, or mouth. A patient might take 1 to 14 days before developing symptoms as corona virus has an incubation period of 14 days. The most common symptoms of COVID-19 include dry cough, tiredness, fever and difficulty breathing (severe cases). Some patients may also suffer from aches and pains, runny nose, nasal congestion, sore throat or diarrhoea.
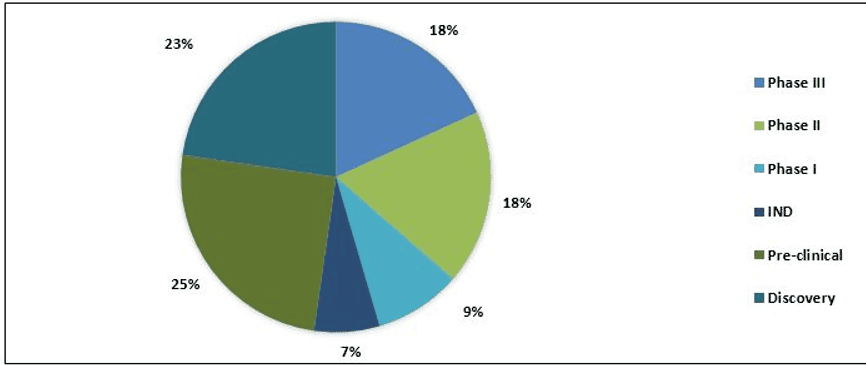
Split of Therapeutic Products
The diagnosis of the disease can be done by several methods such as nucleic acid test, serologic diagnosis and imaging technology. Chest radiograph or CT is an important tool for COVID-19 diagnosis in clinical practice. The majority of Covide-19 cases have similar features on CT images. Currently no antiviral treatment available for SARS-CoV-2 however, companies and research institutes are working towards it. Therapeutic agents targeting nucleosides, nucleotides, viral nucleic acids and enzymes/proteins involved in the replication and transcription of coronaviruses can be promising strategies to treat coronavirus diseases.
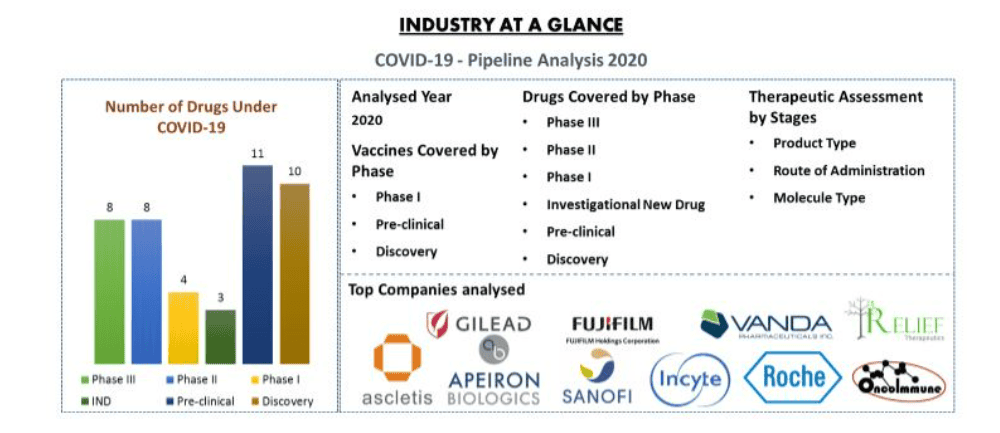
The pipeline of COVID-19 consists of approximately 85+ products in different stages of development. It includes therapeutic drugs and vaccines. Currently, 8+ drugs are in Phase III development and major drugs are in pre-clinical stage. Moreover, 42 vaccines are under development by various companies, and 2+ vaccines are in Phase I trial.
Top Company Analysed
Some of the key players include in the analysis includes Ascletis Pharma Inc., OncoImmune, FUJIFILM Toyama Chemical Co., Ltd.; Sanofi, Gilead Sciences, Incyte Corporation, Hoffmann La Roche, Vanda Pharmaceuticals, Apeiron Biologics and Relief Therapeutics Holdings among others.
Scope of the COVID-19 – Pipeline Analysis 2020:
- The report provides an overview of therapeutic pipeline activity for COVID-19 across the complete product development cycle including all clinical and non-clinical stages
- The report comprises of detailed profiles of COVID-19 therapeutic products with key coverage of developmental activities including licensing & collaboration deals, patents issued, technologies and chemical information
- Therapeutic assessment of the active pipeline products by stage, product type, molecule type, and route of administration
- Detailed profiles of the clinical vaccines and listing of the non-clinical vaccines under development by companies
- Listing of the vaccines under development by universities
Reasons to buy:
- The COVID-19 pipeline report presents the detailed profile of drugs. The analysis offered in the report is a combination of deep dive secondary research and input from Key Opinion Leader of the industry
- The report presents a quick review of the current scenario regarding the drug development of the indication at one glance
- The report covers in-depth analysis of prominent industry peers with a primary focus on company consolidation, technology, agreements and patents regarding the therapy
- Detailed examination on diagnosis and treatment prevailing in the industry
- The study comprehensively covers the market across drugs in different phases of development
Customization Options:
The COVID-19 Pipeline analysis report can be customized to the country level or any other competitive segment. Besides this, UMI understands that you may have your own business need, hence we also provide fully customized solutions to clients.
Table of Content
Research Methodology for COVID-19 – Pipeline Analysis 2020
Overview of pipeline development activities for COVID-19 is not limited to drug description and development activities but also focuses on clinical and pre-clinical results. It also includes company collaboration, licensing deals, funds, and technology and patent details. Therapeutic segmentation of products for COVID-19 is done based on different stage of development. The report comprises of comparative pipeline therapeutics assessment along with detailed drug and vaccine profile of pipeline products by development stage, therapy type, molecule type, and administration route across this indication. Additionally, Executive summary have also been focused upon to give a summary regarding the current market scenario. The detailed profiles of the clinical vaccines products have been elucidated in the complete report and the non-clinical vaccines under development by companies and universities have also been listed.
Seek More Details About Research Methodology
Secondary Research
Detail secondary study was conducted to obtain the COVID-19 Pipeline Analysis through company internal sources such as quarterly reports, performance presentations, press releases, etc., and external sources including trade journals, news & articles, government publications, competitor publications, sector reports, regulatory body’s publications, safety standard organizations, third-party database and other creditable publications.
The secondary research on internal and external sources is being carried out to source qualitative and quantitative information relating to each market.
- Company websites, quarterly reports, financial reports, broker reports, investor presentations and SEC filings
- Industry trade journals and other literature
- National government documents, statistical databases and market reports
- News articles, press releases and web-casts specific to the companies operating in the market
- Several databases for patents and clinical trials
Main objective of the COVID-19 – Pipeline Analysis 2020
The current pipeline trends of the COVID-19 are pinpointed in the study. Investors can gain strategic insights to base their discretion for investments from the qualitative and quantitative analysis performed in the study. Current and future pipeline trends would determine the overall attractiveness of the market, providing a platform for the industrial participant to exploit the untapped market to benefit as first mover advantage. The quantitative objectives for the report include:
- Detailed overview of the indication including etiology, virology, diversity, pathogenesis, signs and symptoms, diagnostic testing and treatment algorithm
- An overview of therapeutic pipeline activity for COVID-19 across the complete product development cycle including all clinical and non-clinical stages
- Analyse the detailed profiles of COVID-19 therapeutic products with key coverage of developmental activities including licensing & collaboration deals, patents issued, technologies and chemical information
- Therapeutic assessment of the clinical pipeline products by stage, product type, molecule type, and route of administration
- Detailed profiles of the clinical vaccines and listing of the non-clinical vaccines under development by companies
- Listing of the vaccines under development by universities
Related Reports
Customers who bought this item also bought