- Home
- About Us
- Industry
- Services
- Reading
- Contact Us
Medical Device Testing Market: Current Analysis and Forecast (2022-2028)
Emphasis on Service Type (Testing Services, Inspection Services, and Certification Services); Testing Type (Physical Testing, Chemical/Biological Testing, Cybersecurity Testing, Microbiology & Sterility Testing, and Others); Phase (Preclinical and Clinical); Product (Active Implant Medical Device, Active Medical Device, Non-Active Medical Device, In-vitro Diagnostics Medical Device, Ophthalmic Medical Device, Orthopedic and Dental Medical Device, Vascular Medical Device, and Others); Sourcing Type (In-House and Outsourced); Device Class (Class I, Class II, and Class III); Region (North America, Europe, Asia-Pacific, Rest of the World); and Region/Country
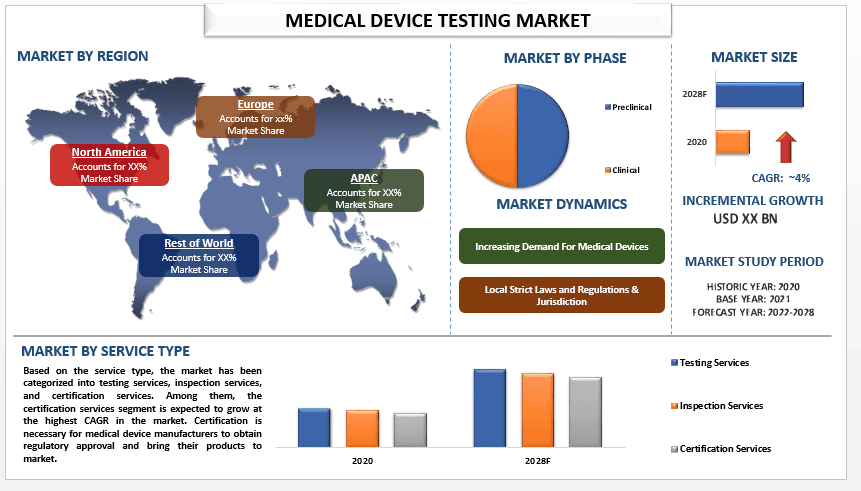
The global medical device testing market is expected to grow at a significant rate of around 4% during the forecast period. The medical device testing market provides services to ensure that medical devices meet regulatory standards and are safe and effective for their intended use. This market includes testing laboratories, certification bodies, and other service providers that offer a range of services such as product testing, quality assurance, compliance testing, and regulatory consulting. The market is driven by increasing regulatory requirements for medical devices, a growing need for product safety and efficacy, and the increasing complexity of medical devices. Regulatory bodies such as the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other national agencies require extensive testing and documentation to ensure that medical devices meet safety and efficacy standards before they can be marketed and sold to healthcare providers and patients.
SGS Société Générale de Surveillance SA, Intertek Group plc, Bureau Veritas S.A., Dekra SE, TÜV SÜD, Eurofins Scientific, British Standards Institution (BSI) Group, Element Materials Technology, UL LLC, and Charles River Laboratories are some of the key players in the market. Several M&As along with partnerships have been undertaken by these players to facilitate customers with hi-tech and innovative products/technologies.
Insights Presented in the Report
“Amongst service type, certification services category to witness higher CAGR during the forecast period”
Based on the service type, the market has been categorized into testing services, inspection services, and certification services. Among them, the certification services segment is expected to grow at the highest CAGR in the market. Certification services are another crucial service type in the medical device testing market. Certification services involve the assessment of medical devices against regulatory standards to ensure that they meet safety and efficacy requirements. Certification is necessary for medical device manufacturers to obtain regulatory approval and bring their products to market.
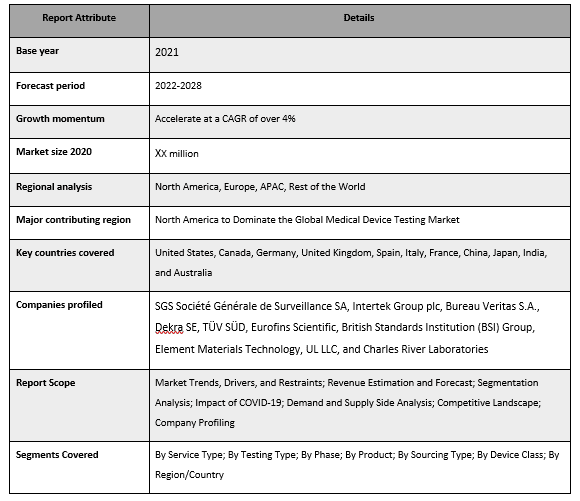
Medical device testing Market Report Coverage
“Amongst testing type, chemical/biological testing segment holds a significant share of the market during the forecast period”
Based on the testing type, the market has been categorized into physical testing, chemical/biological testing, cybersecurity testing, microbiology & sterility testing, and others. Among them, the chemical/biological testing segment holds a significant share of the market in terms of testing type due to its critical role in assessing the compatibility of medical devices with the human body. This type of testing is critical because medical devices often come into contact with body tissues or fluids and can cause harm if they are not compatible with the human body.
“Asia Pacific to witness the fastest growth in the market”
For a better understanding of the market adoption of the medical device testing industry, the market is analyzed based on its worldwide presence in the countries such as North America (United States, Canada, and the Rest of North America), Europe (Germany, France, Italy, Spain, United Kingdom and Rest of Europe), Asia-Pacific (China, Japan, India, Australia, and Rest of APAC), and Rest of World. The Asia-Pacific region is expected to be the fastest-growing region in the medical device testing market due to the increasing demand for medical devices, a growing aging population, and the presence of several key market players in the region. Additionally, the increasing adoption of new technologies such as wireless and mobile medical devices is expected to drive the growth of the medical device testing market in the Asia-Pacific region.
Reasons to buy this report:
- The study includes market sizing and forecasting analysis validated by authenticated key industry experts.
- The report presents a quick review of overall industry performance at one glance.
- The report covers an in-depth analysis of prominent industry peers with a primary focus on key business financials, product portfolio, expansion strategies, and recent developments.
- Detailed examination of drivers, restraints, key trends, and opportunities prevailing in the industry.
- The study comprehensively covers the market across different segments.
- Deep dive regional level analysis of the industry.
Customization Options:
The global medical device testing market can further be customized as per the requirement or any other market segment. Besides this, UMI understands that you may have your own business needs, hence feel free to connect with us to get a report that completely suits your requirements.
Table of Content
Research Methodology for the Medical Device Testing Market Analysis (2022-2028)
Analyzing the historical market, estimating the current market, and forecasting the future market of the global medical device testing market were the three major steps undertaken to create and analyze the adoption of medical device testing in major regions globally. Exhaustive secondary research was conducted to collect the historical market numbers and estimate the current market size. Secondly, to validate these insights, numerous findings and assumptions were taken into consideration. Moreover, exhaustive primary interviews were also conducted, with industry experts across the value chain of the global medical device testing market. Post assumption and validation of market numbers through primary interviews, we employed a top-down/bottom-up approach to forecasting the complete market size. Thereafter, market breakdown and data triangulation methods were adopted to estimate and analyze the market size of segments and sub-segments of the industry pertains to. Detailed methodology is explained below:
Analysis of Historical Market Size
Step 1: In-Depth Study of Secondary Sources:
Detail secondary study was conducted to obtain the historical market size of the medical device testing market through company internal sources such as annual reports & financial statements, performance presentations, press releases, etc., and external sources including journals, news & articles, government publications, competitor publications, sector reports, third-party database, and other credible publications.
Step 2: Market Segmentation:
After obtaining the historical market size of the medical device testing market, we conducted a detailed secondary analysis to gather historical market insights and share for different segments & sub-segments for major regions. Major segments are included in the report as service type, testing type, phase, product, sourcing type, and device class. Further country-level analyses were conducted to evaluate the overall adoption of testing models in that region.
Step 3: Factor Analysis:
After acquiring the historical market size of different segments and sub-segments, we conducted a detailed factor analysis to estimate the current market size of the medical device testing market. Further, we conducted factor analysis using dependent and independent variables such as the service type, testing type, phase, product, sourcing type, and device class of medical device testing. A thorough analysis was conducted for demand and supply-side scenarios considering top partnerships, mergers and acquisitions, business expansion, and product launches in the medical device testing market sector across the globe.
Current Market Size Estimate & Forecast
Current Market Sizing: Based on actionable insights from the above 3 steps, we arrived at the current market size, key players in the global medical device testing market, and market shares of the segments. All the required percentage shares split, and market breakdowns were determined using the above-mentioned secondary approach and were verified through primary interviews.
Estimation & Forecasting: For market estimation and forecast, weights were assigned to different factors including drivers & trends, restraints, and opportunities available for the stakeholders. After analyzing these factors, relevant forecasting techniques i.e., the top-down/bottom-up approach were applied to arrive at the market forecast for 2028 for different segments and sub-segments across the major markets globally. The research methodology adopted to estimate the market size encompasses:
- The industry’s market size, in terms of revenue (USD) and the adoption rate of the medical device testing market across the major markets domestically
- All percentage shares, splits, and breakdowns of market segments and sub-segments
- Key players in the global medical device testing market in terms of products offered. Also, the growth strategies adopted by these players to compete in the fast-growing market
Market Size and Share Validation
Primary Research: In-depth interviews were conducted with the Key Opinion Leaders (KOLs) including Top Level Executives (CXO/VPs, Sales Head, Marketing Head, Operational Head, Regional Head, Country Head, etc.) across major regions. Primary research findings were then summarized, and statistical analysis was performed to prove the stated hypothesis. Inputs from primary research were consolidated with secondary findings, hence turning information into actionable insights.
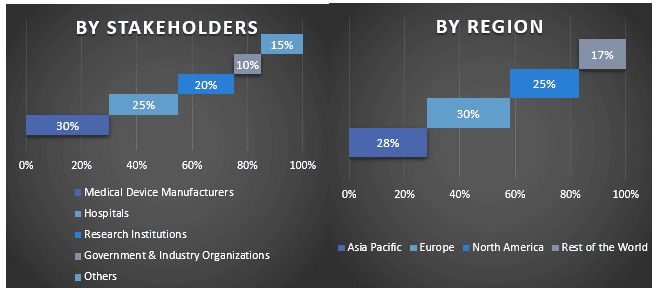
Split of Primary Participants in Different Regions
Market Engineering
The data triangulation technique was employed to complete the overall market estimation and to arrive at precise statistical numbers for each segment and sub-segment of the global medical device testing market. Data was split into several segments & sub-segments post studying various parameters and trends in the service type, testing type, phase, product, sourcing type, and device class in the global medical device testing market.
The Main Objective of the Global Medical Device Testing Market Study
The current & future market trends of the global medical device testing market were pinpointed in the study. Investors can gain strategic insights to base their discretion for investments on the qualitative and quantitative analysis performed in the study. Current and future market trends determined the overall attractiveness of the market at a regional level, providing a platform for the industrial participant to exploit the untapped market to benefit from a first-mover advantage. Other quantitative goals of the studies include:
- Analyze the current and forecast market size of the medical device testing market in terms of value (USD). Also, analyze the current and forecast market size of different segments and sub-segments
- Segments in the study include areas of service type, testing type, phase, product, sourcing type, and device class.
- Define and analysis of the regulatory framework for the medical device testing industry.
- Analyze the value chain involved with the presence of various intermediaries, along with analyzing customer and competitor behaviors of the industry.
- Analyze the current and forecast market size of the medical device testing market for the major region.
- Major countries of regions studied in the report include Asia Pacific, Europe, North America, and the Rest of the World.
- Company profiles of the medical device testing market and the growth strategies adopted by the market players to sustain in the fast-growing market
- Deep dive regional level analysis of the industry
Related Reports
Customers who bought this item also bought